Proteins play a crucial role in the cellular function by adopting specific three-dimensional structures. Protein folding assistants known as chaperonins facilitate the correct folding of newly synthesized proteins into their functional forms. Understanding the mechanisms behind protein folding is essential for unraveling the mysteries of diseases such as Alzheimer’s and Parkinson’s, which are linked to misfolded proteins. Cryo-electron tomography (cryo-ET) has emerged as a powerful tool for visualizing cellular structures in their native environment.
A recent study conducted by researchers at the MPI of Biochemistry in Martinsried and the University Medical Center Göttingen used cryo-ET to investigate chaperonin complexes in the bacterium E. coli. The team focused on monitoring conformational changes in the chaperonins and their interactions with client proteins inside the folding chambers. The results of the study, published in Nature, shed light on the intricate details of the protein folding process with unprecedented clarity.
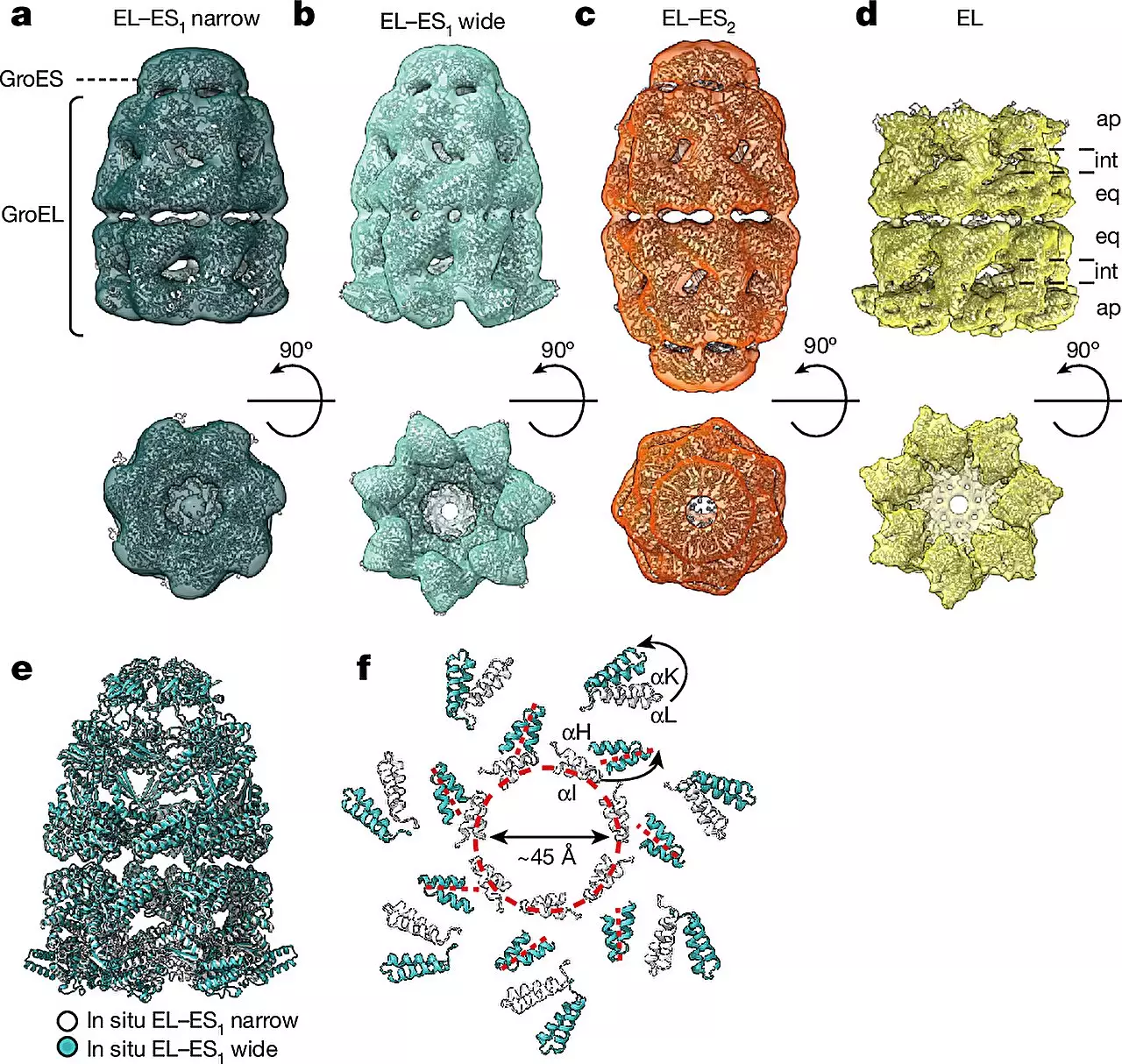
The study revealed two main forms of the GroEL-GroES chaperonin complex, referred to as the “bullet” and “football” shapes. The bullet form, characterized by a GroES cap bound to one side of the GroEL barrel, was predominantly found in bacteria during normal growth. Additionally, football complexes were also observed, showcasing structural symmetry variations within the chaperonin complexes. Microscopic images provided insights into the localization of proteins within the chaperonin barrel during the folding process.
By combining cryo-ET with single-particle cryo-electron microscopy (cryo-EM) and quantitative mass spectrometry, the researchers were able to observe different conformations of chaperonin complexes in various cellular states. This innovative approach allowed for the direct visualization of chaperonin complexes within living cells, a significant breakthrough in the field. The ability to study these complexes in their native cellular environment provides a more accurate representation of their functions compared to traditional in vitro experiments.
The findings from the study suggest that chaperonins undergo distinct assembly patterns and transition between the bullet and football shapes during the protein folding process. This dynamic behavior highlights the complexity of chaperonin-assisted folding and underscores the importance of studying these mechanisms in a cellular context. The researchers’ ability to capture detailed images of protein folding processes opens up new avenues for investigating the roles of chaperonins in health and disease.
Cryo-electron tomography has revolutionized the study of cellular structures, enabling researchers to explore the intricate processes involved in protein folding. The insights gained from the recent study on chaperonin complexes in bacteria emphasize the importance of understanding the mechanisms underlying protein folding for developing novel therapeutic strategies for protein misfolding diseases. By harnessing the power of advanced imaging techniques, scientists are pushing the boundaries of knowledge in molecular biology and paving the way for new discoveries in the field of structural biology.