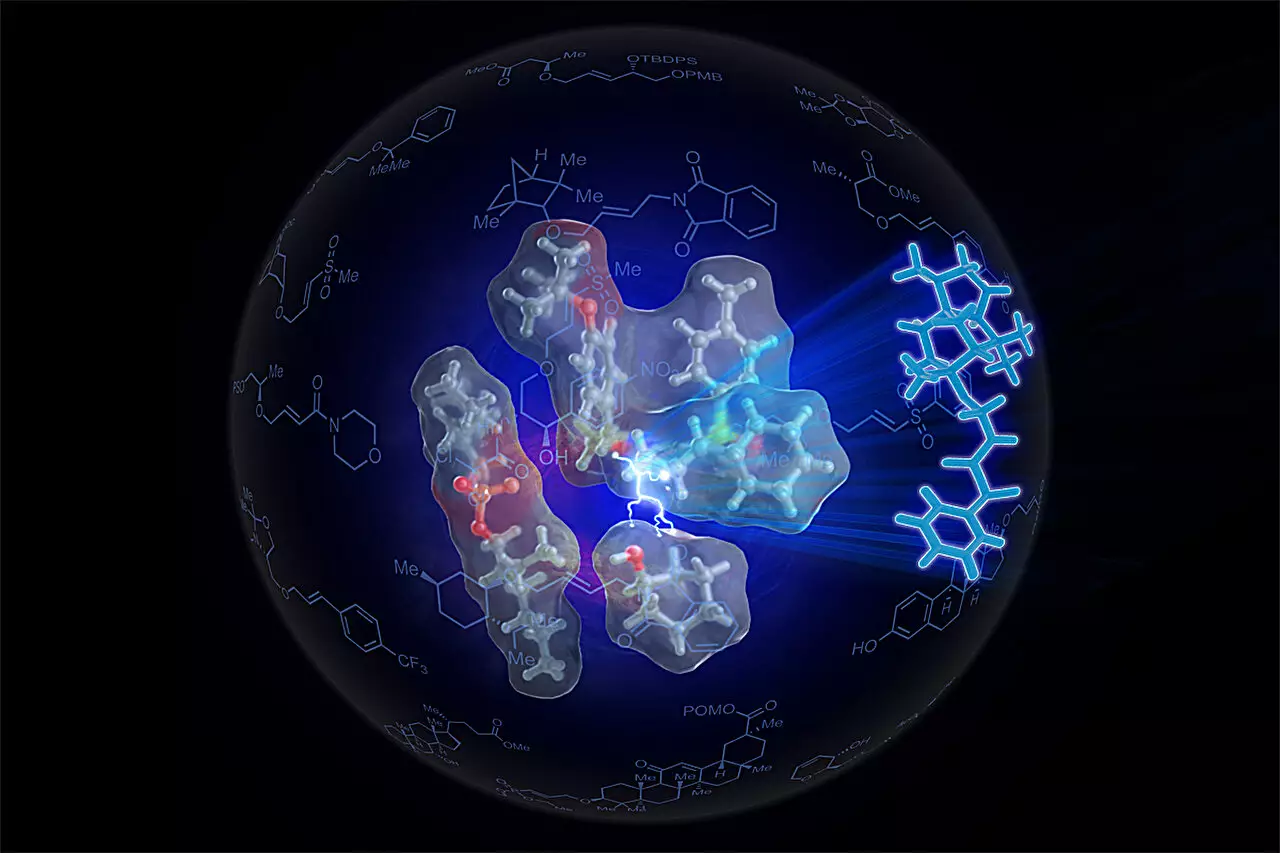
In an era where efficiency and sustainability are paramount, researchers at the University of Illinois Urbana-Champaign have made a revolutionary leap in the synthesis of ethers, vital components in the pharmaceutical, food, and personal care industries. Led by Professor M. Christina White, the team has engineered a new type of catalyst that streamlines the synthesis process, drawing inspiration from biological enzymes that orchestrate complex chemical reactions with precision.
Ethers are ubiquitous in various applications, from medication to everyday consumer goods, yet their traditional synthesis has posed significant challenges. The conventional method requires activating an alcohol by removing a proton, which transforms it into a reactive entity. However, this process generates a mix of unwanted by-products, complicating the purification of the desired product. Additionally, these procedures demand substantial quantities of raw materials, creating inefficiencies particularly detrimental to the creation of complex and valuable molecular structures. Graduate student Sven Kaster, the study’s lead author, aptly noted that the existing methods are not practical for the synthesis of intricate compounds.
In their quest for a more effective approach, the researchers took a page from nature’s playbook. Enzymes are known for catalyzing reactions by positioning reactants closely and favorably, facilitating interactions that would otherwise be improbable. Inspired by this biological mechanism, White and her team sought to mimic the configurational advantages enzymes offer during chemical reactions. They devised a catalyst dubbed SOX, integrated with palladium, which facilitates a bond cleavage between carbon and hydrogen in alkenes, thus enabling their reaction with alcohols without the need for prior activation.
Introducing Sven-SOX: The Game Changer
Recognizing that presenting reactants in optimal proximity and orientation was crucial for successful ether production, the team further refined their catalyst into what they named Sven-SOX. This iteration boasts a meticulously designed geometry and specific electronic properties, ensuring that the activated alkene and the alcohol align perfectly during the reaction. Professor White provided an illustrative analogy: “Two people wanting to hold hands not only need to be close but must also face each other the right way.” This is the essence of the researcher’s innovation—creating an artificial “enzyme” from simple components that can coax chemical components into favorable interactions.
Expansive Applications and Benefits
The ramifications of this new methodology are profound. With the Sven-SOX catalyst, the team produced over 130 unique ethers, including various complex molecules previously thought to be exceptionally difficult to synthesize. Kaster highlighted the versatility of their approach: “We can create many ethers that have not been synthesized before, potentially possessing novel properties.” This flexibility not only diminishes the wastage of materials but also demonstrates the capacity of the catalyst to handle sensitive groups that traditional methods often undermine.
Moreover, the procedure has been refined to such an extent that it could feasibly be performed at an elementary education level. By utilizing mild conditions and reducing the quantity of materials required, the researchers have effectively democratized the synthesis process, showcasing how scientific innovation can lead to both practical and educational benefits.
Looking ahead, the research team aims to explore other small-molecule catalysts that could replicate the extraordinary efficiency seen in enzyme catalysis for a wider variety of chemicals. This pursuit not only opens the door to further innovations in ether synthesis but also paves the way for breakthroughs in the synthesis of numerous other organic compounds. White remarked on the broader implications of their work, emphasizing how it underlines the significance of foundational research and the untapped potential of small molecules to exhibit enzyme-like behavior.
Conclusion: A Testament to Nature’s Wisdom
The remarkable advancements in ether synthesis at the University of Illinois Urbana-Champaign underscore the profound impact that nature can have on scientific innovation. By observing and emulating biological systems, researchers are not only solving practical problems but also illuminating paths for future inquiries into catalysis and synthetic chemistry. This innovative stride in synthesis techniques is poised to redefine how a myriad of essential chemical compounds can be produced, ultimately benefiting diverse sectors reliant on complex organic molecules. The integration of bio-inspired design principles into chemistry holds vast promise, proving that the answers to some of our most pressing scientific challenges may well lie within nature’s existing solutions.