In the intricate world of molecular biology, helical structures underpin the functionality of many critical biomolecules, particularly proteins. These helices emerge from the precisely ordered arrangement of their constituent amino acids—fundamental units that define the protein’s characteristics and capabilities. A deeper grasp of helix formation not only illuminates their structural importance but also paves the way for exploring the diverse biological behaviors of proteins throughout various biological systems.
One of the fascinating properties of helices is chirality, which refers to the geometric property of objects that are not superimposable on their mirror images. Chirality significantly influences how helical structures are organized and operates within the realm of peptides, which are essentially shorter segments of proteins. As researchers have demonstrated, the specific sequence and arrangement of amino acids in a peptide determine the resultant chiral helix. This phenomenon hints at a more profound layer of informational complexity that may dictate peptide interactions, stability, and overall functionality.
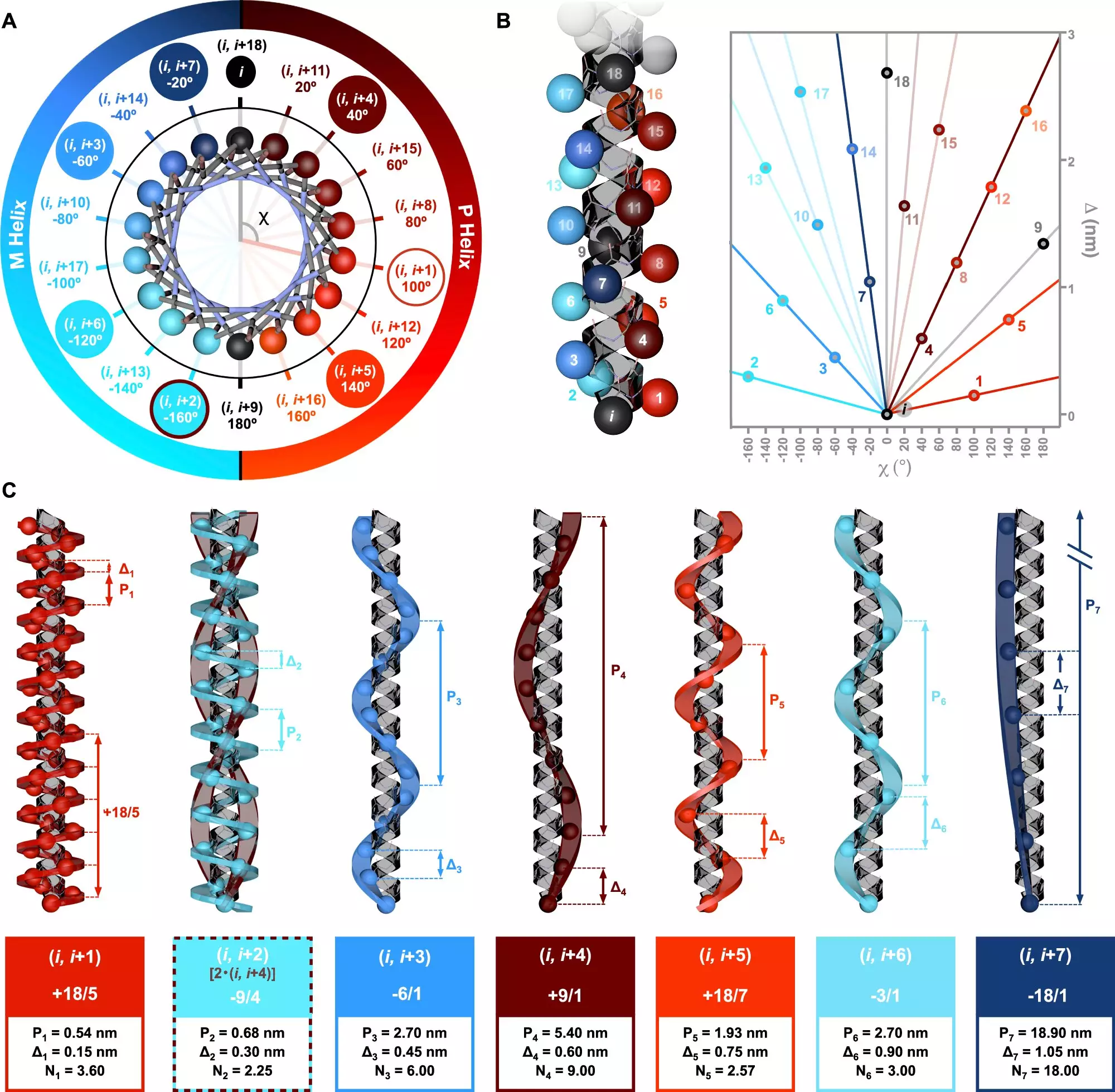
A pioneering study published in *Nature Communications* sheds light on the advancements in our understanding of helical structures, particularly in alpha-helices. Led by Dr. Julián Bergueiro at the Center for Research in Biological Chemistry and Molecular Materials (CiQUS), the research team utilized a combination of computational simulations and cutting-edge circularly polarized light spectroscopy. This dual approach allowed the researchers to delve into how varying amino acid sequences give rise to distinct helical conformations. The discovery of a new symmetry model has broadened our understanding of how these molecular architectures interact with chiral light, leading to insightful revelations about peptide structure relationships.
The research findings have implications that extend far beyond academic curiosity; they open avenues for new technological applications. The ability to strategically control the sequence of monomers enables scientists to engineer specific helical topologies along polymer chains. This capability holds significant promise for the design of innovative molecules in various sectors, including medicine, where tailored peptides can lead to improved drug delivery systems or novel therapeutic agents. Additionally, the creation of biocompatible materials through synthetic peptides could revolutionize numerous applications in biotechnology.
This groundbreaking research serves as a pivotal moment in peptide chemistry, reshaping our comprehension of helical structures and their functional roles. As scientists continue to unravel the complexities of helical formations, the potential for harnessing these insights in practical applications becomes increasingly tangible. The ongoing exploration of peptide helices will undoubtedly contribute to more refined understanding and manipulation of biological molecules, paving the way for advancements in both scientific research and applied technologies.