In the ongoing battle against climate change, the ability to recycle carbon dioxide (CO2) into usable fuels is becoming a crucial area of research. This transformation, known as the artificial photosynthesis process, aims to create sustainable energy solutions by repurposing waste carbon emissions. Notably, researchers from the University of Michigan have made significant strides in this arena, showcasing an innovative system capable of binding carbon atoms to produce ethylene, a valuable hydrocarbon. This article delves into the technology, methodology, and implications of their groundbreaking work.
The University of Michigan’s artificial photosynthesis system stands out due to its efficiency and durability in producing ethylene. This hydrocarbon, widely utilized in manufacturing plastics, typically relies on fossil fuels, exacerbating CO2 emissions during production. The Michigan team has demonstrated performance levels that are approximately five to six times superior to other existing technologies in the realm of solar energy-driven CO2 reduction.
Zetian Mi, a professor in electrical and computer engineering at the university, emphasizes the importance of ethylene, noting that it is the most produced organic compound globally. Traditional methods of ethylene production involve high temperatures and pressures, resulting in significant carbon emissions. The Michigan team’s approach, however, endeavors not only to create ethylene sustainably but also to mitigate the attendant environmental impacts.
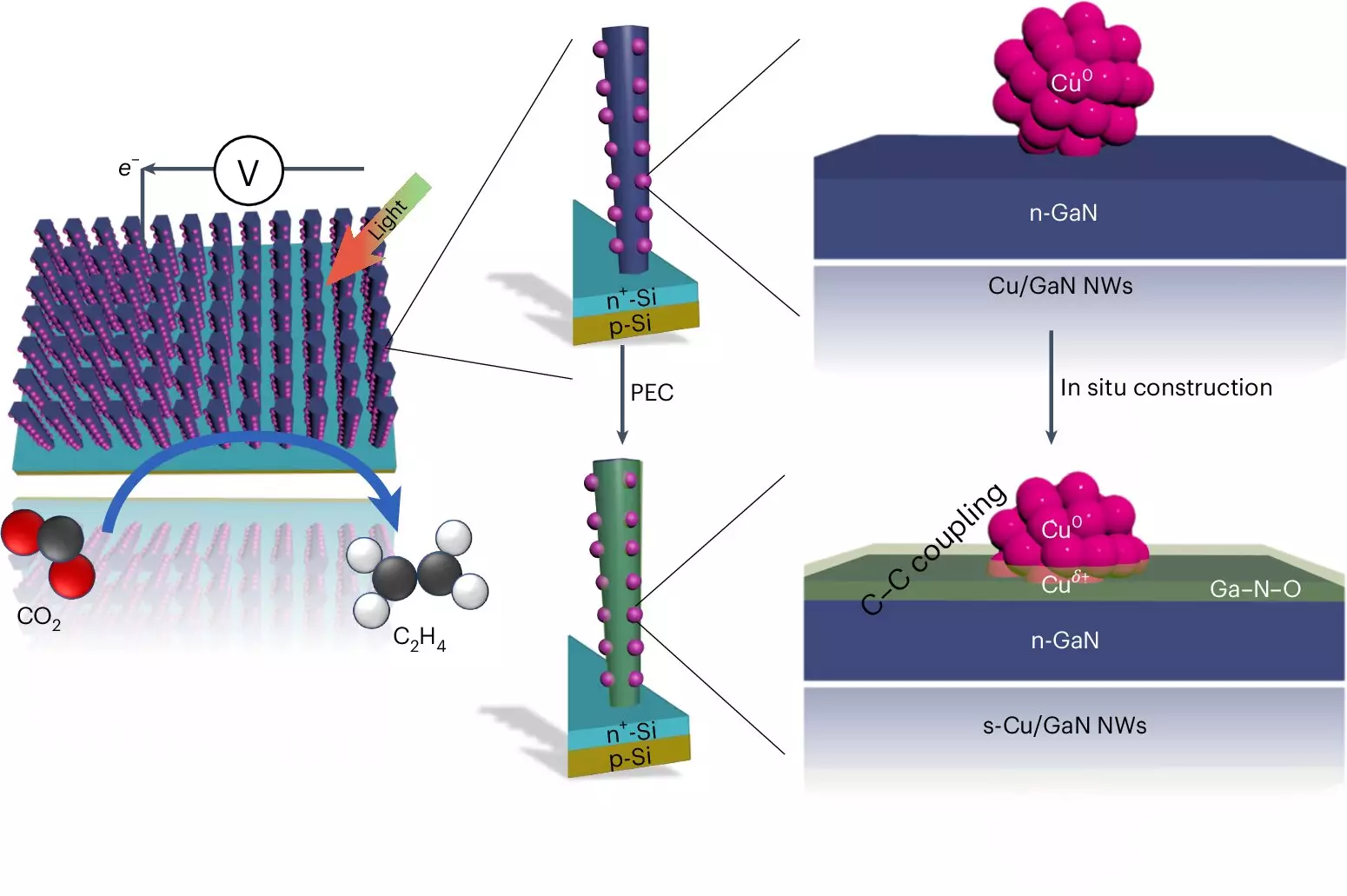
At the heart of this artificial photosynthesis system lies a combination of advanced materials and chemical processes. The device harnesses the power of light through two distinct types of semiconductors: gallium nitride nanowires and a silicon base. The nanowires, extremely small at approximately 50 nanometers in width, are submerged in a CO2-enriched water solution. When exposed to sunlight, the energy excites electrons, leading to chemical reactions essential for producing ethylene.
During the process, water molecules are split near the surface of the gallium nitride nanowires, yielding hydrogen while the corresponding oxygen is absorbed by the gallium nitride, transforming into gallium nitride oxide. Copper clusters, strategically placed on the nanowires, play a critical role by binding to both hydrogen and carbon from the CO2. This innovative design allows for the transformation of carbon monoxide into ethylene through a well-orchestrated interaction at the interface between the copper and the gallium oxide.
The efficiency of the system is compelling. The research indicated that 61% of the free electrons generated contribute to the reaction for ethylene production. While similar catalytic systems based on silver and copper demonstrate comparable efficiencies, they require carbon-based fluids and have limited operational lives—typically just a few hours. In stark contrast, the Michigan system has displayed remarkable endurance, functioning continuously for an impressive 116 hours, and has already been tested in longer durations of up to 3,000 hours.
This exceptional performance can be attributed, in part, to the beneficial relationship between gallium nitride and the water-splitting process. The oxygen produced from this reaction enhances the catalyst’s efficiency while enabling a self-healing capability that protects the system from degradation.
Although the immediate success of this artificial photosynthesis system is encouraging, the research team’s ultimate ambitions extend beyond ethylene production. They aim to develop higher-chain hydrocarbons and liquid fuels—substances that could revolutionize transportation technologies and significantly contribute to sustainable energy practices. As Bingxing Zhang, a researcher involved in the project, points out, the goal is to explore the production of multicarbon compounds such as propanol, which has substantial potential for fuel applications.
By focusing on the synthesis of liquid fuels that can be easily transported and utilized within existing technology frameworks, the team’s research could have far-reaching implications for reducing dependency on fossil fuels and curbing greenhouse gas emissions.
The advancements made at the University of Michigan in artificial photosynthesis represent an exciting leap toward addressing a critical environmental challenge. By converting CO2 emissions into useful fuels like ethylene, researchers are paving the way for innovative pathways to support sustainability. As this field evolves, the potential to produce liquid fuels could fundamentally alter the energy landscape, offering a promise for cleaner, greener alternatives to conventional fossil fuels. The intersection of technology, science, and the drive for a more sustainable future is indeed illuminated by these pioneering efforts.