In a significant advancement for sustainable organic synthesis, a team of chemists from the National University of Singapore (NUS) has unveiled an innovative iron-catalyzed method to synthesize trisubstituted Z-alkenes. Detailed in the journal *Nature Synthesis*, this cutting-edge technique addresses the longstanding challenge associated with the formation of these important chemical compounds. Trisubstituted alkenes are fundamental building blocks in numerous biologically active molecules and are vital in a range of stereospecific reactions that lead to the creation of sp3-hybridized structures.
The Challenge of Selectivity in Alkene Synthesis
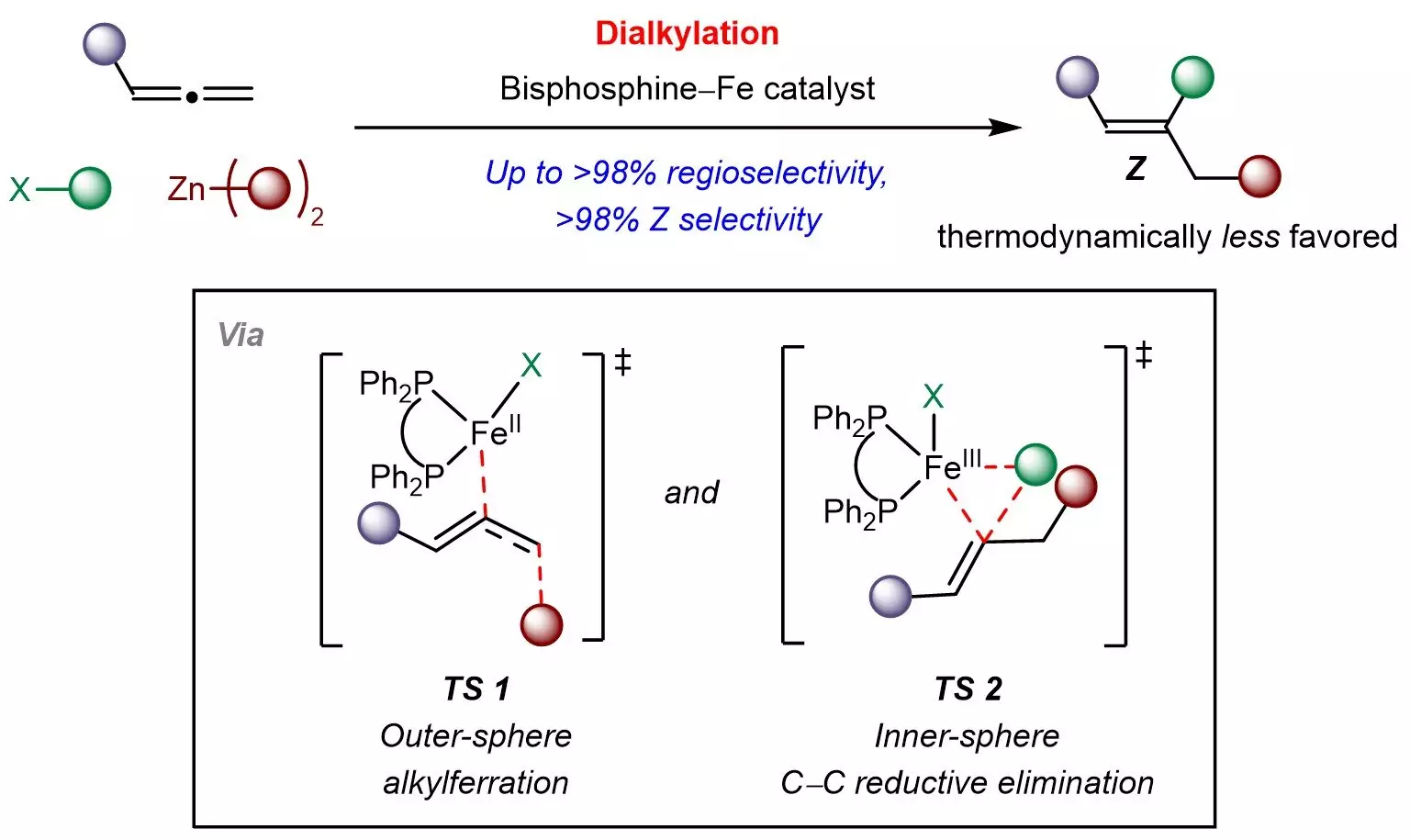
The synthesis of Z-alkenes typically presents hurdles primarily due to their lower thermodynamic stability compared to their E-isomers. This presents a difficulty in selectively producing the Z-isomers, which are often required for their unique chemical properties. The innovative method conceived by the team from NUS utilizes a kinetically controlled catalytic process that challenges this stability issue, thus providing a new pathway for the synthesis of these complex molecules.
Innovation Through Iron Catalysis
At the heart of the NUS team’s approach is a cost-effective and readily accessible bisphosphine-iron catalyst. This catalyst is pivotal in facilitating the reaction between allenes and various readily available chemical building blocks, including sp3-hybridized organohalides and organozinc reagents. Through their multicomponent strategy, researchers can introduce diverse aliphatic groups into the allene framework, carefully managing both the site of reaction and the resultant Z-selectivity.
What sets this method apart is the utilization of iron, a non-toxic, abundant, and economically viable transition metal, reinforcing the method’s alignment with environmentally friendly practices. Such a green approach not only lowers the economic barriers to accessing these compounds but highlights the potential for broader applications in the field of organic chemistry.
The implications of this groundbreaking research extend beyond mere academic interest. The team has effectively applied their method in the development of a glucosylceramide synthase inhibitor, which contains a crucial trisubstituted Z-alkene. The Z-configuration in this case is essential for maintaining the bioactivity of the compound, emphasizing the practical relevance of the team’s work in drug discovery and therapeutic development.
Collaborating with experts from The Chinese University of Hong Kong and the Agency for Science, Technology and Research (A*STAR), the NUS team further elucidated a distinctive reaction mechanism involving outer-sphere radical-mediated alkylferration followed by inner-sphere carbon-carbon bond formation. This insight offers valuable direction for designing other kinetically controlled reactions involving allenes and similar π-systems.
Looking ahead, the research team at NUS is set to apply the insights gained from this study to develop additional multicomponent transformations that not only optimize the use of readily available raw materials but also convert them into valuable chemical products with industrial potential. This research paves the way for future studies that could enhance the efficiency and sustainability of chemical manufacturing processes, ultimately contributing to a greener and more sustainable future in organic synthesis.