Lithium and sodium are not just any elements; they are at the heart of a significant transformation in battery technology. These alkali metals promise to enhance the performance of solid-state batteries, which are gaining traction for their superior energy density, safety, and longevity compared to traditional lithium-ion batteries. As the push for more efficient and sustainable energy storage solutions continues, insights into the microstructures of lithium and sodium anodes offer exciting possibilities for battery innovation. The detailed understanding of how these metals behave and how their structures are formed during battery operation is paramount to overcoming existing challenges and unlocking the full potential of solid-state batteries.
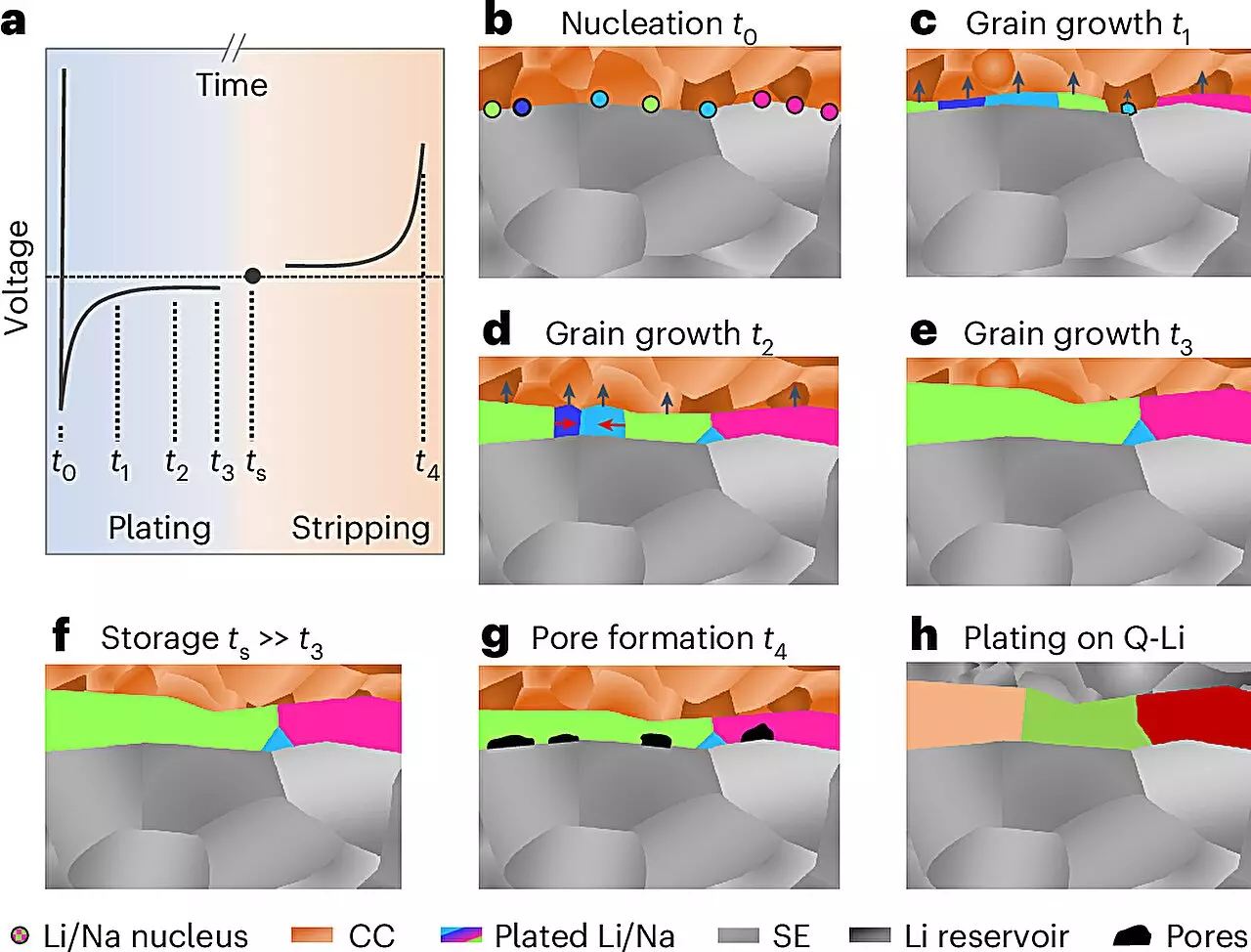
Historically, the electrochemical properties of metals have been well-studied, leading to innovations in their applications across various technologies. However, the unique reactivity of lithium and sodium has posed a substantial barrier to revealing their microstructural properties. Chemical reactivity results in the rapid formation of surface layers that obscure our understanding of their internal structures. This has led to a notable knowledge gap compared to other metals commonly used in industry. A recent collaborative effort led by researchers at Justus Liebig University Giessen (JLU), alongside experts from the University of California, Santa Barbara, and the University of Waterloo, has finally cracked this code. By developing a meticulous methodology under highly controlled conditions, they have successfully characterized the microstructure of electrochemically deposited lithium and sodium for the first time.
The innovative approach devised by the research team involves a sequence of low-temperature preparation processes and analyses performed in inert gas environments. This technique culminates in the use of electron backscatter diffraction to visualize the internal structures of the metals in unprecedented detail. With this advanced methodology, precise observations about the microstructures of lithium and sodium layers—some reaching up to 100 micrometers in thickness—were achieved. Prof. Dr. Jürgen Janek, leading the research efforts at JLU, remarked on the surprising grain sizes of the resulting layers. His findings are expected to significantly influence ongoing research initiatives, particularly within the POLiS (Post Lithium Energy Storage) excellence cluster focused on exploring alternative energy storage solutions.
Despite the promise held by lithium and sodium anodes, achieving practical applications within solid-state batteries is not without its hurdles. The structural integrity of metal electrodes during electrochemical cycling is a major concern, as these metals tend to deform under charge and discharge conditions. This deformation can lead to the formation of unwanted pores and dendritic structures, which increase the risk of short circuits—a critical safety issue in battery operation. The research aims to mitigate these challenges by developing methods that allow for metal formation only during specific phases of the charging process, thereby reducing handling difficulties associated with highly reactive metal foils.
A Collaborative Effort in Materials Science
The journey to uncover the microstructural secrets of lithium and sodium wouldn’t have been possible without extensive collaborative efforts. Prof. Janek’s seasoned partnerships with the research groups from Santa Barbara and Waterloo have culminated in significant progress in the field. The intricacies of material compositions, electrode preparation, and analytical techniques required a diverse array of expertise, showcasing the value of interdisciplinary cooperation in scientific research. The breakthroughs in imaging and characterizing the microstructures of lithium and sodium electrodes represent a monumental step, not just for academic research, but for the future of solid-state battery implementations.
With the foundational knowledge gained from this research, the next steps involve translating these insights into practical advancements in solid-state battery technology. The understanding of electrochemical processes and structural formation mechanisms can guide researchers in tailoring battery performance, efficiency, and safety. As countries push for sustainable energy solutions, the development of high-performance solid-state batteries with lithium and sodium metal anodes could lead to substantial changes in how we store and utilize energy. The findings published in reputable scientific journals underline the potential for further exploration and could pave the way for next-generation battery technologies that address existing environmental concerns while meeting the growing demand for energy storage systems.
The research into the microstructures of lithium and sodium metal offers thrilling prospects for the battery industry. As innovations unfold, the electrochemical storage landscape will likely evolve, highlighting the importance of ongoing investigations and collaboration in advancing technology. Understanding the complexities of these alkali metals is not merely an academic pursuit; it holds the key to revolutionizing energy systems for a more sustainable future.