The electrochemical reduction of carbon dioxide (CO2) is emerging as a pivotal technology in the quest to mitigate climate change and transform CO2 into valuable chemical feedstocks. While significant progress has been made in enhancing catalyst efficiency through innovative designs and modifications, the influence of the electrolyte composition has often been underestimated. Recent breakthrough research indicates that varying the electrolyte not only affects the efficiency of the reaction but also significantly alters the product output, presenting a new frontier in catalytic science.
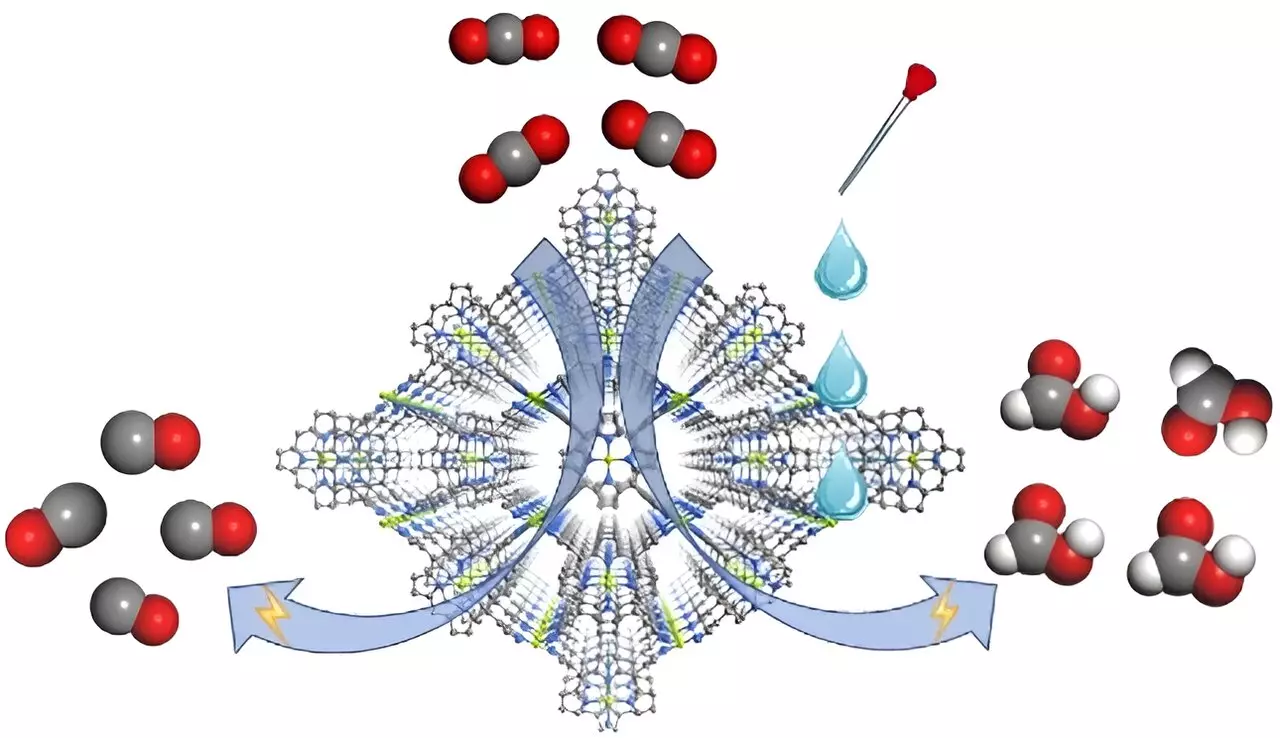
In a groundbreaking study featured in Angewandte Chemie International Edition, a team led by prominent scientists from the Fujian Institute of Research on the Structure of Matter has pushed the boundaries of conventional research. They focused on a metal-organic framework (MOF) catalyst, specifically designated as FICN-8. This innovative catalyst is designed using Cu(porphyrin)-derived ligands alongside Cu(pyrazolate) components, culminating in a three-dimensional porous architecture. Such structural characteristics dramatically enhance substrate accessibility to the active metalloporphyrin sites, facilitating a more efficient CO2 reduction process.
Unlike traditional approaches predominantly focused on catalyst refinement, this study uniquely highlights the critical role of electrolyte variations in determining the nature of the reduced products. In tests conducted with tetrabutylammonium hexafluorophosphate (TBAPF6) in acetonitrile, FICN-8 demonstrated remarkable efficacy, achieving a CO production selectivity of up to 95%. However, the introduction of proton sources, such as water or trifluoroethanol (TFE), pivoted the main product away from CO towards formic acid. This adaptability highlights an important tunable aspect of the electrochemical processes, where systematic manipulation of the electrolyte can lead to different reaction pathways.
The research team undertook a series of deliberate experiments aimed at unraveling the complex mechanisms driving product selectivity. Their kinetic isotope effect (KIE) measurements revealed a stark contrast between the pathways for CO and formic acid production. The near-identity KIE value for CO indicated a less dependency on protons in its formation, whereas a substantial KIE of approximately 3.7 for formic acid suggested a direct proton involvement. These insights were further supported by theoretical calculations pinpointing hydride (*H) adsorption on the porphyrin nitrogen site as a trigger for formic acid generation, whereas CO formation followed a proton-independent route.
The implications of this research are significant, suggesting that the design of electrocatalytic systems must encompass the interplay between catalyst structure and electrolyte composition. This opens avenues for creating novel catalyst-electrolyte combinations that could enhance the output of value-added products from CO2 reduction processes. As we advance towards a sustainable future, understanding and exploiting these relationships will be crucial in optimizing CO2 utilization technologies further.
The findings underscore a transformative perspective on electrochemical CO2 reduction, illustrating that product selectivity may not solely hinge on the catalyst’s design but significantly on the environment in which it operates. As researchers continue to explore this domain, the significance of electrolyte considerations will likely become a central theme in the development of next-generation catalysts.