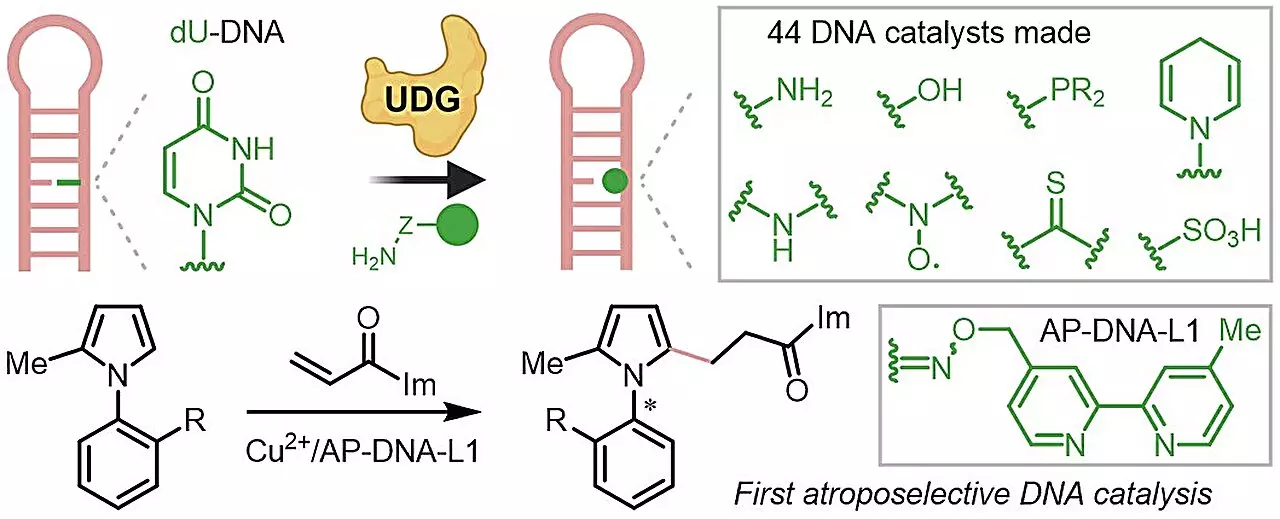
In a remarkable stride in the field of asymmetric catalysis, researchers at the National University of Singapore (NUS) have unveiled novel techniques for synthesizing chiral DNA catalysts. This innovation stems from the ingenious integration of DNA repair processes with biorthogonal chemistry, significantly enhancing the efficiency and versatility of catalytic methods. The importance of chiral molecules in pharmaceuticals and materials science underlines the potential impact of this research.
Traditional enzyme catalysis, while effective, has limitations. Biological proteins, though natural catalysts, exhibit instability and require complex design processes to manipulate their functions. This study provides a solution by repurposing DNA as a stable and cost-effective alternative. Unlike proteins, DNA boasts a unique base-pairing capacity that allows for easy programmability, offering meticulous control over its structural characteristics and functionalities.
The research spearheaded by Assistant Professor Zhu Ru-Yi has advanced methods that simplify the production of DNA catalysts. This simplicity means that even those lacking specialized skills can engage in DNA catalysis without reliance on sophisticated equipment. This democratization of technology is a notable advancement, as it opens the door for broader applications across various fields, potentially revolutionizing the way chiral compounds are synthesized.
A critical benefit of the approach lies in its compatibility with bioorthogonal reactions, which allow for the introduction of various functional groups without interference with the DNA’s properties. This compatibility expands the horizons for the types of reactions that can be performed, offering researchers greater flexibility and a plethora of options in catalyst design.
The NUS team successfully crafted a library of 44 distinct DNA catalysts using this innovative technique, each excelling in enantioselectivity and overall reaction efficiency. Perhaps the most groundbreaking accomplishment was the demonstration of atroposelective DNA catalysis, enabling the generation of chiral compounds that are notoriously challenging to produce through existing biocatalytic methods.
This pioneering work not only highlights the robustness of the new method but also showcases the ability to create diverse catalysts with unprotected functional groups—adding another layer of complexity and utility to the toolkit of chemists.
Looking forward, the research team is poised to explore additional strategies for advancing selective and sustainable chemical reactions utilizing DNA catalysis. This initiative not only promises to push the boundaries of current methodologies but also aims to make significant contributions to the fields of synthetic biology and green chemistry.
The findings published in the Journal of the American Chemical Society mark a significant milestone in the evolution of asymmetric catalysis, positioning DNA as a crucial player in the synthetic chemistry landscape. The potential implications of this research extend beyond academic curiosity; they herald new possibilities for more sustainable and efficient practices in pharmaceuticals and material sciences.