A recent publication by a research team led by Professor Jaeheung Cho from the Department of Chemistry at UNIST represents a significant leap in our understanding of the interactions between cobalt(III)-based metal complexes and nitrile compounds. This study, featured in the esteemed Journal of the American Chemical Society, explores the intricate reaction mechanisms involved in the activation of nitriles, a class of compounds crucial for drug development and environmental applications. This investigation paves the way toward overcoming some of the long-standing challenges associated with the reactivity of nitriles in chemical processes.
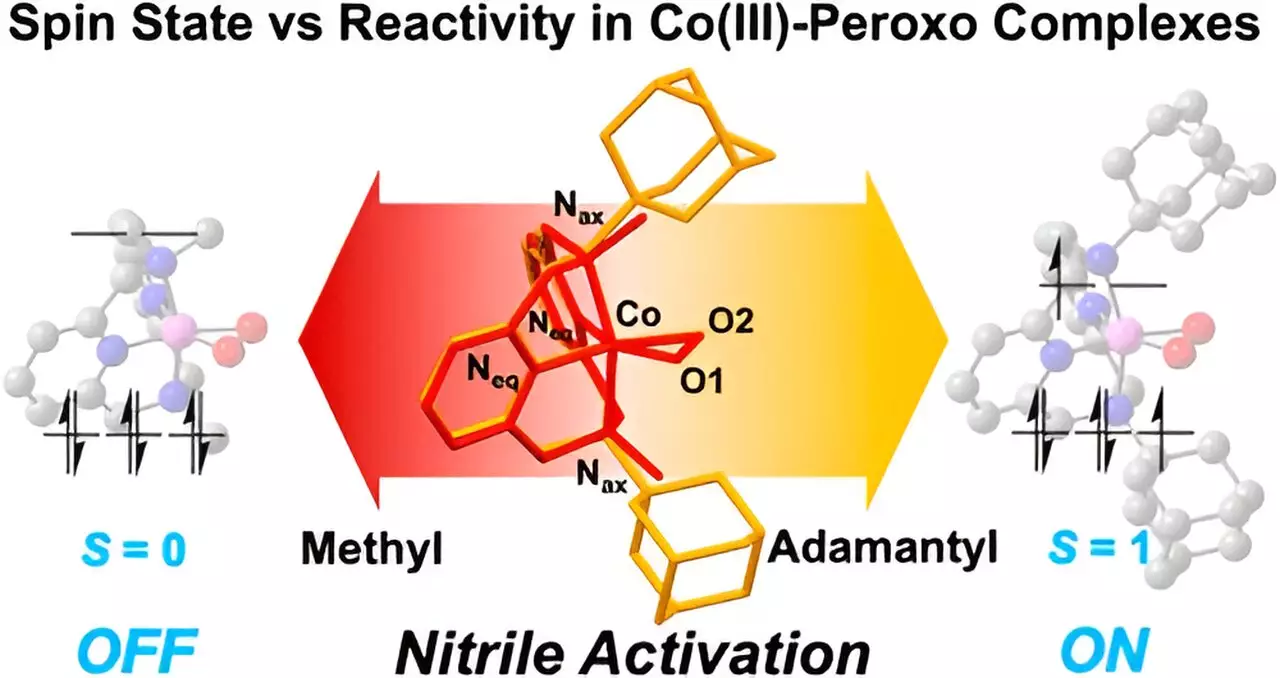
One of the standout revelations from the study is the critical role that metal spin states play in the reactivity of cobalt complexes. The researchers illustrated that relatively minor alterations in the properties of cobalt can lead to substantial changes in the rates and products of the reactions. This finding underscores the complexity of metal-ligand interactions—a cornerstone of coordination chemistry—and its implications for optimizing chemical reactions in synthetic pathways. Understanding these properties may present opportunities for the development of novel compounds with enhanced efficacy.
The research team employed a sophisticated experimental approach, utilizing the “Macrocyclic Pyridinophane System.” This structure allowed them to tailor the cobalt compounds systematically, providing insights into how different structural features influence reaction outcomes. Notably, the presence of larger adamantyl groups resulted in pronounced nitrile activation, while smaller functional groups like methyl showed negligible reactivity. This differential behavior underscores the intricate balance between structural configuration and electronic effects, a factor that chemists must consider when designing new metallodrugs.
Implications for Pharmaceuticals
Nitriles hold significant importance within the pharmaceutical and agricultural sectors. However, their inherent reactivity challenges have often hindered their application, necessitating the discovery of more efficient activation methods. The study’s findings about the cobalt(III)-peroxo species reveal a promising pathway for generating nitrile reactions at room temperature, suggesting potential utility in anticancer drug development. As first author Seonghan Kim articulated, the connections revealed in their findings between cobalt spin states and nitrile reactivity hold exciting prospects for future research.
Professor Cho’s research marks a pivotal point in both inorganic chemistry and therapeutic development. By elucidating the foundational mechanisms governing cobalt(III)-nitrile interactions, this study not only enriches the scientific community’s understanding but also hints at exciting applications in drug discovery and material sciences. The implications of this research could contribute significantly to the design of new and more effective chemical agents, fostering advancements in treating diseases and enhancing environmental safety. This innovative approach might just herald a new era for pharmaceuticals, where the mechanistic insights gleaned from such studies enable the synthesis of complex compounds that can meet modern therapeutic demands.