Industrial emissions are a major contributor to environmental pollution, and mitigating their adverse effects is vital for public health and ecological preservation. The focus on reducing toxic nitrogen oxides (NOx)—specifically nitric oxide (NO) and nitrous oxide (N2O)—has led researchers to investigate the capabilities of zeolite-based catalysts. Recent findings from the Paul Scherrer Institute (PSI) in collaboration with CASALE SA have shed light on the mechanisms through which these catalysts operate, highlighting the pivotal role of iron atoms within their nanoporous structures.
The Importance of Eliminating Nitrogen Oxides
Nitrogen oxides are harmful gases produced in various industrial processes, including the manufacture of fertilizers. More than just a byproduct, both NO and N2O pose substantial health risks, contributing to respiratory diseases and environmental degradation. Nitrous oxide is particularly notable for its potent greenhouse effects, as it is nearly 300 times more effective than carbon dioxide at trapping heat in the atmosphere. Consequently, industries are compelled to implement methods for the effective removal of these gases from their emissions, and zeolite-based catalysts offer promising solutions.
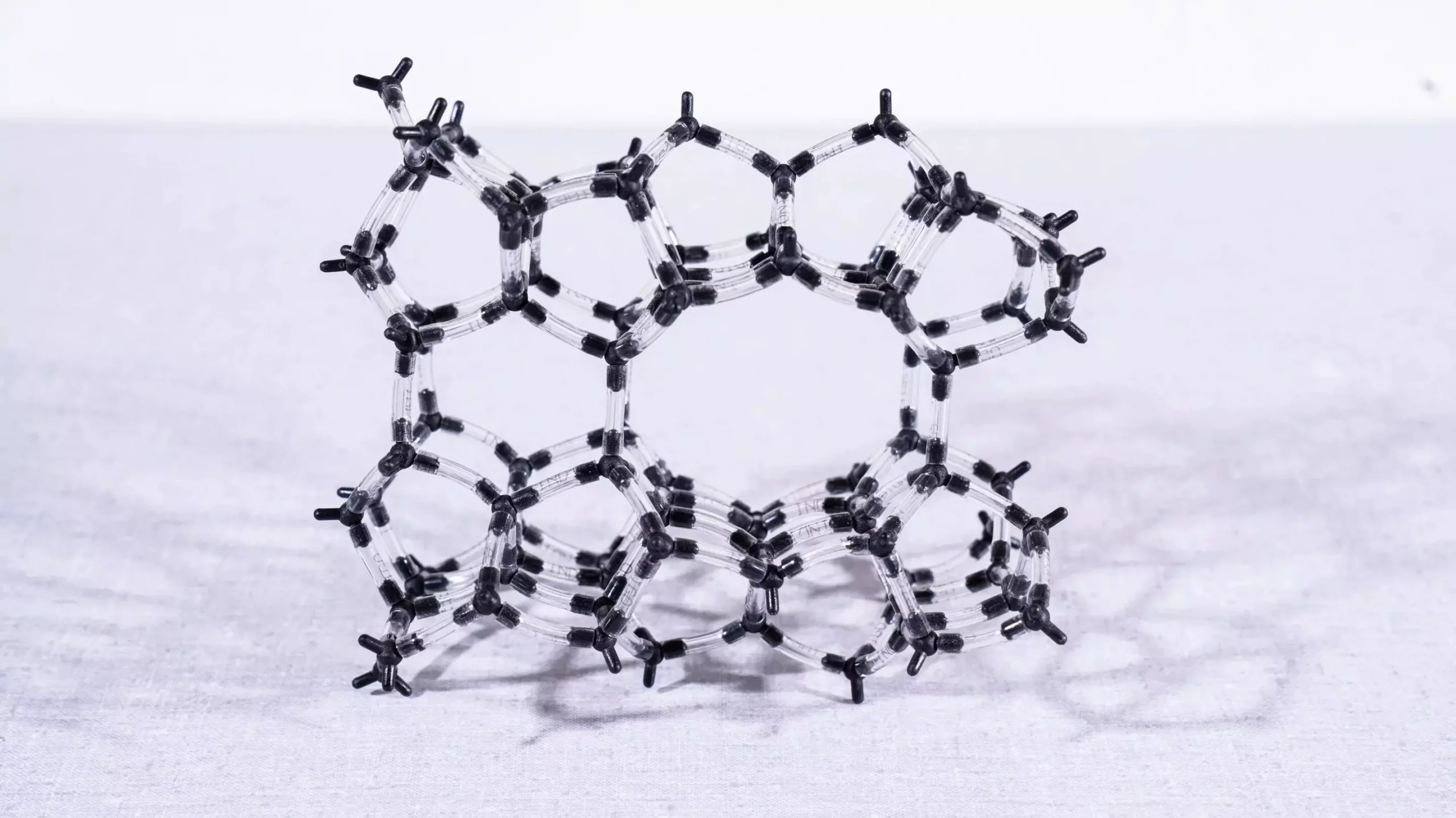
Zeolites are crystalline aluminosilicates characterized by an intricate porous structure that allows for selective catalytic activity. Their framework, primarily composed of aluminum, oxygen, and silicon, can be synthetically engineered or derived from natural minerals. When iron atoms are incorporated, they become active sites for catalyzing chemical reactions—most notably the conversion of harmful nitrogen oxides into benign substances. Yet, the complexity of these catalysts, with iron atoms present in various forms and arrangements, raises questions about their specific contributions to catalysis.
Researchers, led by Davide Ferri and doctoral candidate Filippo Buttignol, aimed to decode how the positioning and state of iron within the zeolite impact its catalytic efficacy. Understanding these relationships is crucial for enhancing the performance of zeolite catalysts in industrial applications.
The research team utilized an array of advanced spectroscopic techniques to investigate the behavior of iron species during the catalytic reaction in real-time. By analyzing zeolite samples at the Swiss Light Source (SLS), they employed X-ray absorption spectroscopy to observe all iron species simultaneously. This comprehensive approach was complemented by electron paramagnetic resonance spectroscopy, which allowed for the differentiation of the contributions from individual species, and infrared spectroscopy to explore molecular aspects of these iron configurations.
Through these combined methodologies, the study revealed critical insights: catalytic activity is maximized when iron occurs in pairs located at specific neighboring sites within the zeolite lattice. This interaction forms the basis for effective NOx removal, as one iron atom facilitates the conversion of N2O while the other processes NO. The research underscores the importance of the precise spatial arrangement of these active sites, which can significantly enhance the efficiency of nitrogen oxide abatement.
The findings elucidate that the effectiveness of zeolite-based catalysts is contingent upon the fine-tuning of iron location and configuration. As Ferri suggests, identifying the precise sites for catalysis provides a foundation for engineering improved catalysts. This understanding could lead to tailored manufacturing processes that optimize the arrangement of iron within the zeolite framework, thereby increasing the overall efficiency of NOx removal.
As industries face increasing regulations to reduce emissions and pollutants, the insights from PSI’s research will play a crucial role in shaping future catalyst design. The ability to convert toxic nitrogen oxides into harmless molecules will not only benefit air quality and public health but also contribute positively towards mitigating climate change.
The advancement of zeolite-based catalysts opens new avenues in the quest to reduce harmful emissions from industrial operations. With the support of rigorous scientific inquiry, researchers at the Paul Scherrer Institute have illuminated the complexities of catalytic processes, revealing how the arrangement of iron within zeolite frameworks is integral to converting toxic nitrogen oxides. As science continues to blur the lines between chemistry and environmental stewardship, this research stands to inform best practices for cleaner, more sustainable industrial processes. In the face of a growing environmental crisis, such innovations are not merely beneficial—they are essential.