Cholesterol has long been a subject of scientific inquiry due to its fundamental role in the structure and function of cell membranes. Researchers from Rice University, under the guidance of Jason Hafner, have recently made strides in this field, promising new insights into how cholesterol operates within cellular environments. The findings, published in the Journal of Physical Chemistry, focus on unraveling the complex interaction between cholesterol and the membranes that house cellular receptors, opening potential avenues for future research directed at diseases such as cancer, where membrane integrity is essential.
Cell membranes are intricate matrices composed of lipids and proteins, with cholesterol serving as a crucial component. However, the challenge for researchers has been understanding how cholesterol is structured and interacts with its membrane environment. Jason Hafner, a distinguished professor in both physics and chemistry, emphasizes the significance of this study, asserting that comprehending membrane organization can catalyze advancements in dissecting the mechanisms behind various diseases. The research team’s approach—a combination of laser-based spectroscopy and theoretical modeling—represents a significant leap in this complex area.
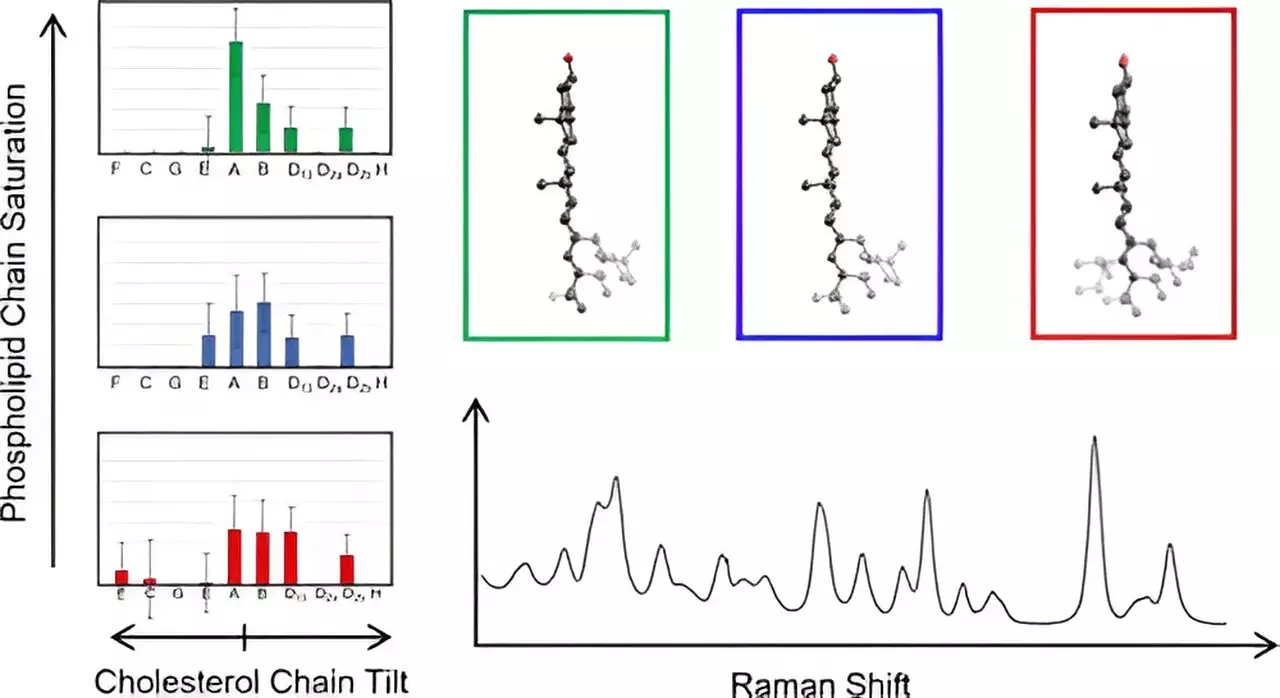
To navigate the complexities associated with cholesterol characterization, Hafner’s lab employed Raman spectroscopy, an advanced technique that involves scattering laser light to yield detailed vibrational spectra of molecules. This method provided the researchers with unprecedented insights into cholesterol’s structural variability within membranes. By analyzing spectra from cholesterol molecules in various configurations and juxtaposing them with computational models derived from density functional theory (a sophisticated quantum mechanical method), they gleaned essential information on cholesterol’s unique properties, specifically its fused ring structure and attached carbon chains.
In a remarkable discovery for the field, the researchers analyzed 60 distinct cholesterol configurations, revealing that these structures could be grouped based on the alignment of their carbon chains relative to the ring structure. Such observations highlight previously unrecognized variations in cholesterol organization, propelling the understanding of its role in cellular contexts forward. It was particularly striking for the team to find uniform low-frequency spectra across grouped cholesterol molecules, which not only facilitated a more straightforward analysis but also helped map cholesterol chain structures in their native membrane habitats.
The implications of this study extend beyond mere academic curiosity; they pave the way for further investigations into cellular dysfunctions linked to membrane organization. As Hafner aptly notes, this research could significantly influence the understanding of diseases tied to cell membrane dynamics, particularly cancer. The collaboration between graduate students and ongoing researchers bolsters the study’s credibility and showcases the vibrant scientific community at Rice University. Future studies built upon these insights may yield innovative strategies to address health challenges tied to cell membrane integrity and cholesterol’s multifaceted role, promising a healthier future driven by scientific advancement.