Biological research often gravitates towards the well-trodden paths of protein folding, gene expression, and neurotransmission. For good reason, these processes are integral to our understanding of cellular function and disease development. However, a recent shift in focus has spotlighted a less conventional aspect of cellular dynamics: biological condensates. These complex structures, akin to oil droplets dispersed in water, emerge based on density differences within the cellular environment, and they challenge our traditional views of intracellular organization.
Biological condensates are fascinating entities within cells, emerging without the need for membranes that characterize other cellular compartments. These condensates can unify or separate different proteins and molecules, which influences their behavior and function—sometimes propelling reactions and other times impeding them. Despite previous discoveries about their local effects, particularly their ability to provide alternative energy sources, the broader implications of these structures have remained largely unexplored until now.
Recent findings from researchers at Duke University and Washington University in St. Louis reveal that biological condensates extend their influence far beyond their immediate surroundings within the cell. Their work, published in the journal *Cell*, underscores the potential of these structures as regulators of intracellular electrochemistry, significantly impacting how cells interact with their environment.
The groundbreaking research suggests that biological condensates function almost like wireless transmitters, relaying signals that modify cellular behavior. Lingchong You, a key figure in this study, articulates that these structures can facilitate alterations in a cell’s electrical properties, which might seem counterintuitive to their relatively innocuous appearance. This has profound implications for cellular processes that depend on the precise balancing of ionic charges, such as the interaction with antibiotics.
As condensates form, they have the capacity to absorb various biomolecules while excluding others. This selective filtration can create charged environments that resonate throughout the cell, thereby initiating far-reaching effects on cellular physiology. Yifan Dai, another researcher involved in this study, emphasized that even a handful of these condensates can trigger cascading effects, redefining the understanding of gene regulation and cellular response mechanisms.
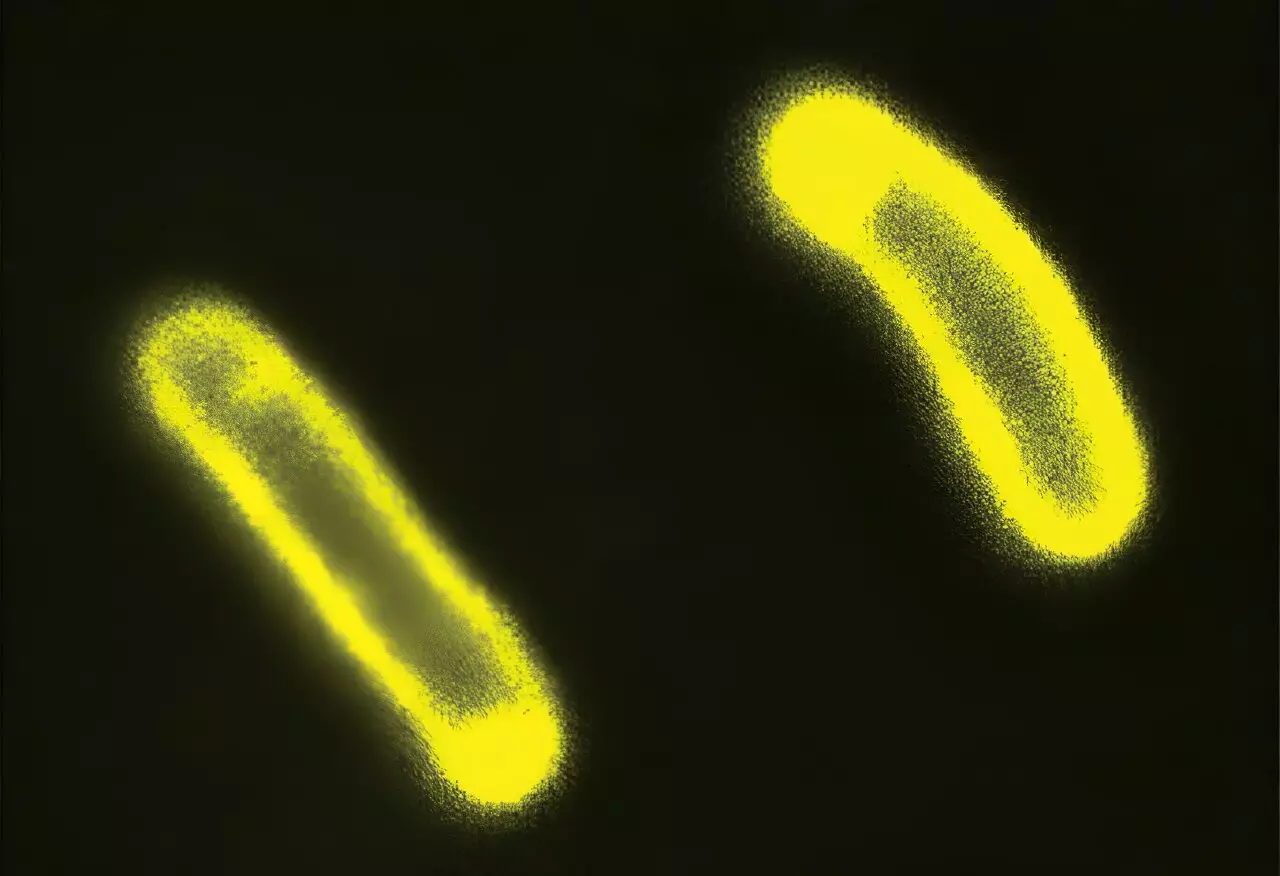
To illustrate their findings, the researchers conducted experiments involving *E. coli* bacteria. By inducing the formation of condensates within these bacterial cells—either through physical stress or genetic manipulation—they observed shifts in the electrical charge of cellular membranes. This alteration directly correlated with the bacteria’s response to various antibiotics. The results indicated that certain antibiotics could be more effective or less so depending on the electrochemical milieu generated by the presence of condensates.
This discovery hints at a novel mechanism of antibiotic resistance, driven not by genetic mutations but by the dynamic internal state of the bacteria influenced by condensates. The ability of these structures to modify cellular environments presents a paradigm shift in our approach to studying microbial behavior and understanding how cells develop resistance to treatment.
The implications of this research extend beyond mere curiosity about condensates. They open new avenues for understanding fundamental biological processes and disease resistance mechanisms. As Ashutosh Chilkoti suggests, this is “likely just the tip of the iceberg.” The electrical potential created by condensates could have vast effects on various cellular activities, including metabolic processes, signaling pathways, and evolutionary adaptations.
Given the intricate interactions between biological condensates and cellular functionality, future research should aim to dissect these relationships further. Investigating how condensates influence not only bacterial survival but also eukaryotic cells could yield insights into fundamental cellular behavior.
The discovery of biological condensates as significant players in maintaining cellular integrity and function challenges long-standing perceptions of cellular architecture. As researchers delve deeper into their roles, the potential to redefine our understanding of cellular processes broadens, promising avenues for innovative therapeutic strategies and a more comprehensive grasp of life’s complex biochemical networks. The exploration of these “unassuming blobs” holds the key to unlocking myriad biological secrets that could benefit both basic science and medical advancements.