Aluminum oxide, chemically represented as Al2O3, is an intriguing material with a diverse array of applications. Commonly referred to as alumina, corundum, or even as gemstones like sapphire and ruby, this substance is much more than its aesthetic appeal. Aluminum oxide is renowned for its insulating properties, rendering it invaluable in electronics, chemical resistance applications, and as a support medium in catalytic processes. Understanding the surface properties of alumina is crucial, especially when it comes to optimizing its performance in various chemical reactions and catalytic applications.
The Challenge of Surface Structures
Despite its significance, the study of aluminum oxide has been complicated by the insulating characteristics of the material. For over fifty years, scientists struggled to gain a precise understanding of its surface structure, which is critical for comprehending how reactions occur on its surface. The internal atomic arrangement of aluminum oxide is orderly, but the surface structure deviates, presenting a significant research challenge. The complexity of determining the surface arrangement has led to its notoriety as one of the “three mysteries of surface science” since it remained unresolved until recent advancements in experimental methods.
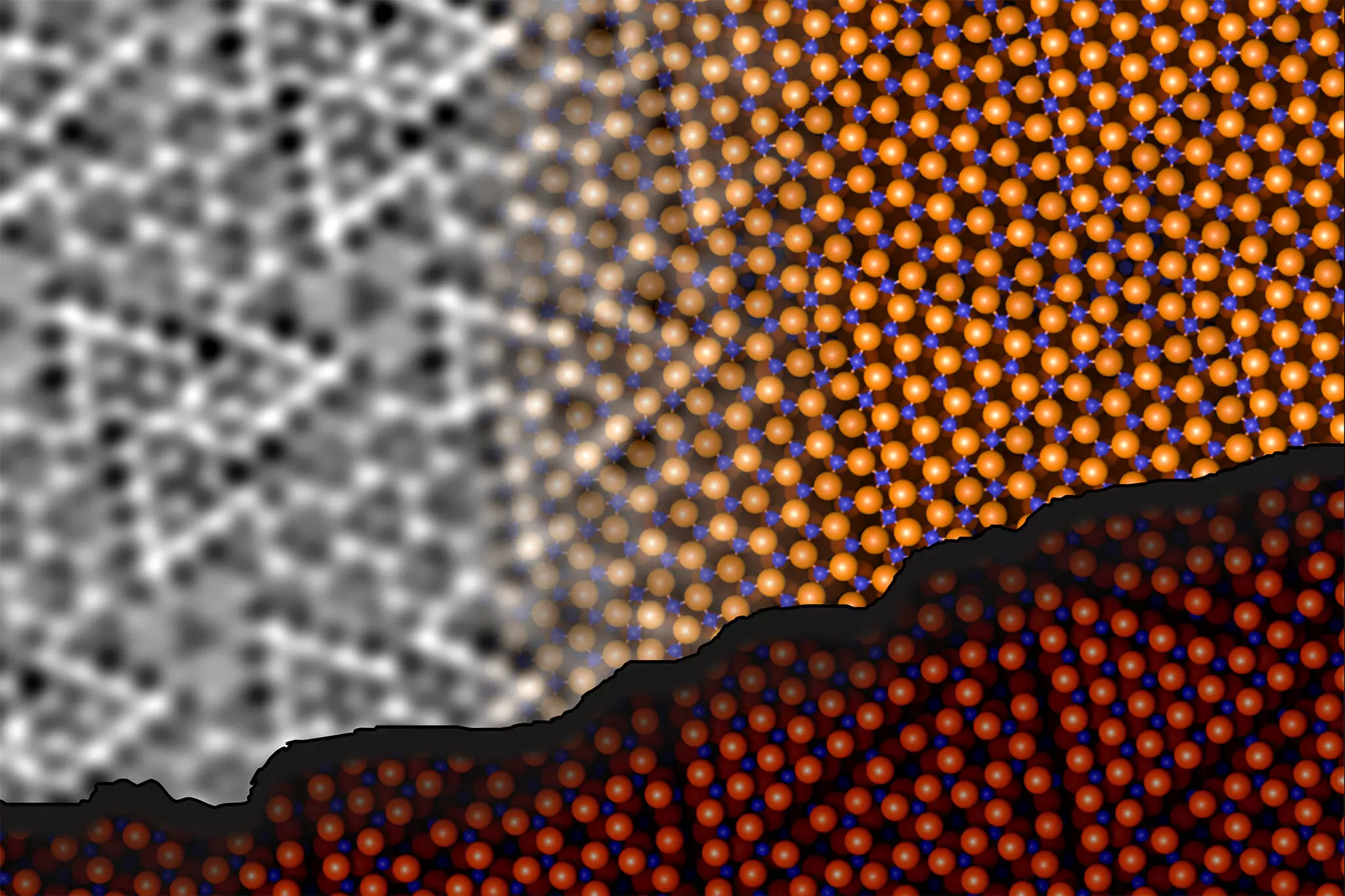
A collaborative research team from TU Wien and the University of Vienna made remarkable strides in unveiling this mystery, publishing their findings in the prestigious journal *Science*. Led by researchers Jan Balajka and Ulrike Diebold, the team successfully analyzed aluminum oxide’s surface using a novel technique called noncontact atomic force microscopy (ncAFM). This cutting-edge method allows for the observation of surface structures by scanning a finely pointed tip near the surface without direct contact. The non-invasive nature of this technique opens new avenues for research, allowing scientists to bypass the limitations posed by the strong insulating properties of aluminum oxide.
In their approach, the researchers tackled the challenge of chemical sensitivity, which has long impeded the identification of specific surface atoms. Using an ingenious tactic, they attached a single oxygen atom to the tip of the ncAFM apparatus. This innovative setup enabled them to differentiate between oxygen and aluminum atoms when mapping the surface. The interactions between the tip and the surface atoms revealed the intricate chemistry underlying the material. By meticulously controlling the tip’s movement, they could visualize the precise organization of the atoms based on local repulsion and attraction forces, a breakthrough that allows for better understanding of aluminum oxide’s unique properties.
The results of their research revealed a significant finding: aluminum atoms on the surface do not merely sit idle; instead, they form chemical bonds with oxygen atoms located in the deeper layers of the material. This dynamic rearrangement stabilizes the overall structure of aluminum oxide, contrary to previous beliefs suggesting a fixed atomic ratio of aluminum to oxygen. The findings contribute to a paradigm shift in how scientists understand the interactions at play within this enigmatic insulator.
The complexity of the surface structure was further unraveled by employing advanced machine learning algorithms, which played a crucial role in modeling the structure effectively. The integration of computational methods with experimental observations provided the researchers with a comprehensive view of the underlying crystalline arrangement. Andrea Conti, who undertook the computational modeling, expressed the importance of these methodologies in overcoming the challenges posed by the abundant structural possibilities.
The joint efforts of both experimental and computational research not only demystified the structure of aluminum oxide but also unveiled design principles pertinent to a wider array of materials. This breakthrough paves the way for future advancements in fields such as catalysis and material science, promising to enhance various applications involving aluminum oxide and its derivatives. As the complexity of material behavior continues to unfold, insights gained from this seminal research will undoubtedly guide the next generation of innovations within the scientific community. The pursuit of knowledge in material science remains vibrant, with each discovery laying the groundwork for enhanced functionality and efficiency in technology.