The impact of carbon dioxide (CO2) emissions on global warming is garnering increasing attention as scientists and environmentalists seek solutions to mitigate climate change. Among the various strategies proposed, the carbonation of cement-based materials emerges as a promising approach, not only for capturing CO2 but also for transforming it into stable mineral forms. The research conducted by a team led by Associate Professor Takahiro Ohkubo at Chiba University delves into the intricacies of this process, focusing on the conditions that enhance the carbonation mechanism within cement. Their investigation, published in The Journal of Physical Chemistry C, sheds light on the factors influencing this vital chemical reaction and its practical implications.
At its core, the carbonation process in cement paste starts with the dissolution of CO2 in water, which in turn interacts with calcium silicate hydrates (C–S–H) produced during the hydration of cement. This interaction converts dissolved CO2 into carbonate ions, which then react with calcium ions to form stable calcium carbonate. However, while the fundamental chemistry is relatively straightforward, the detailed mechanisms remain poorly understood—a gap that has led to extensive research efforts within scientific communities.
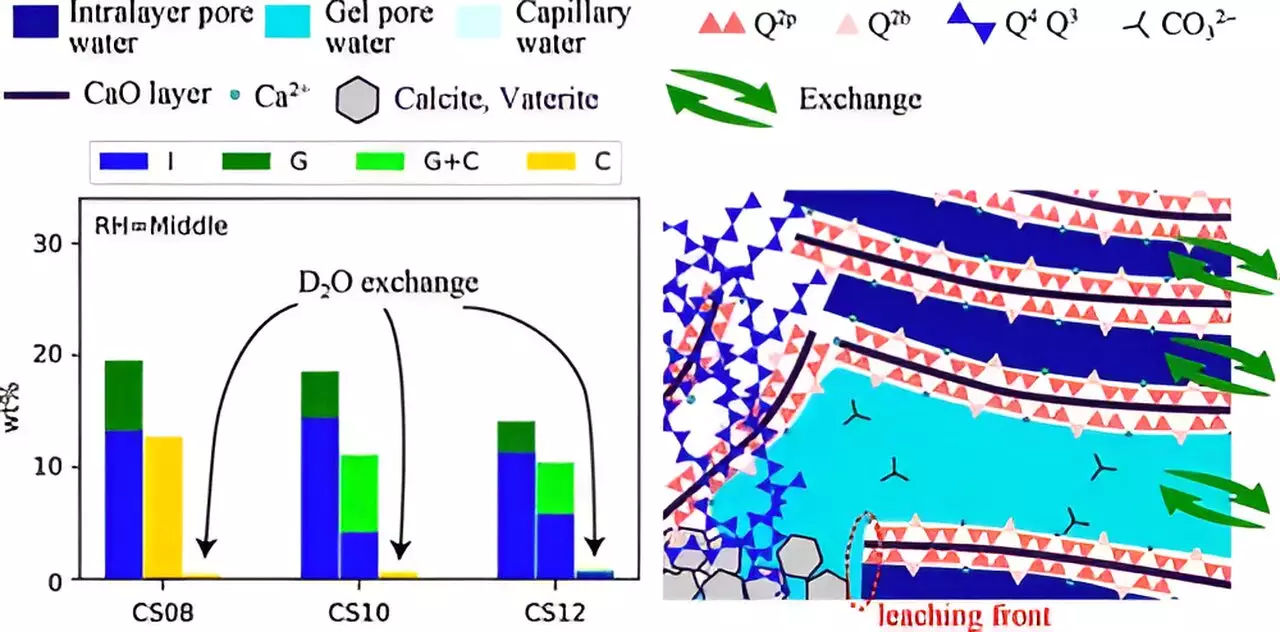
Research has indicated that several variables significantly impact carbonation, including relative humidity (RH), CO2 solubility, and the calcium to silicon (Ca/Si) ratio prevalent in C–S–H. Understanding the transport of ions and water through the nanostructured gel-pore water globally present in C–S–H is equally crucial. Through their innovative study, Ohkubo and his team aim to provide clarity on how these various factors interplay during carbonation.
To explore the carbonation process more thoroughly, the researchers relied on advanced methods including 29Si nuclear magnetic resonance (NMR) and 1H NMR relaxometry. These techniques allow for an enhanced understanding of water transport within C–S–H, shedding light on structural modifications during carbonation. The team synthesized C–S–H samples and exposed them to accelerated carbonation using 100% CO2, which is significantly higher than what cement materials typically experience in natural environments over decades. This approach allows for quicker analysis while still yielding insights relevant to real-world conditions.
Experiments were conducted under varying levels of relative humidity and different Ca/Si ratios, providing a comprehensive dataset that reveals how structural changes uniquely manifest under different conditions.
The results of the study illustrated that carbonation leads to notable changes in the structural composition of C–S–H, such as the collapse of its chain structure and modifications in pore size. Importantly, the research identified that lower humidity and a higher Ca/Si ratio correlated with smaller pore sizes, which in turn inhibited the leaching of Ca2+ ions and water—a crucial factor that contributes to inefficient carbonation.
The findings underscore the synthesis of structural adjustments along with mass transfer processes as a critical aspect of the carbonation reaction. This holistic view goes beyond simply observing how structure changes in response to carbonation; it emphasizes the need to understand how mass transfer links to structural integrity and reactivity.
Ohkubo and his team suggest that their research could pave the way for developing new building materials capable of sequestering substantial amounts of atmospheric CO2. The implications of their study extend beyond the construction industry, as carbonation is also prevalent in organic compounds within natural ecosystems. By elucidating the foundational processes behind carbonation in cement, the team’s approach may help tackle broader carbon capture challenges, thereby contributing to environmental sustainability on multiple fronts.
The study conducted by Ohkubo and his collaborators represents a significant stride in our understanding of the mechanisms governing carbonation in cement-based materials. By identifying the key variables that affect this process, they have clarified how modifications impact CO2 absorption, thereby positioning this research as a cornerstone for future innovations in eco-friendly construction practices. As the world grapples with the adverse effects of climate change, advancing our ability to capture and utilize CO2 will be vital for developing sustainable solutions. The insights gained from this research not only enhance our technical grasp of carbonation mechanisms but also spotlight the potential for transforming how we think about CO2 emissions and material design in the pursuit of environmental stewardship.