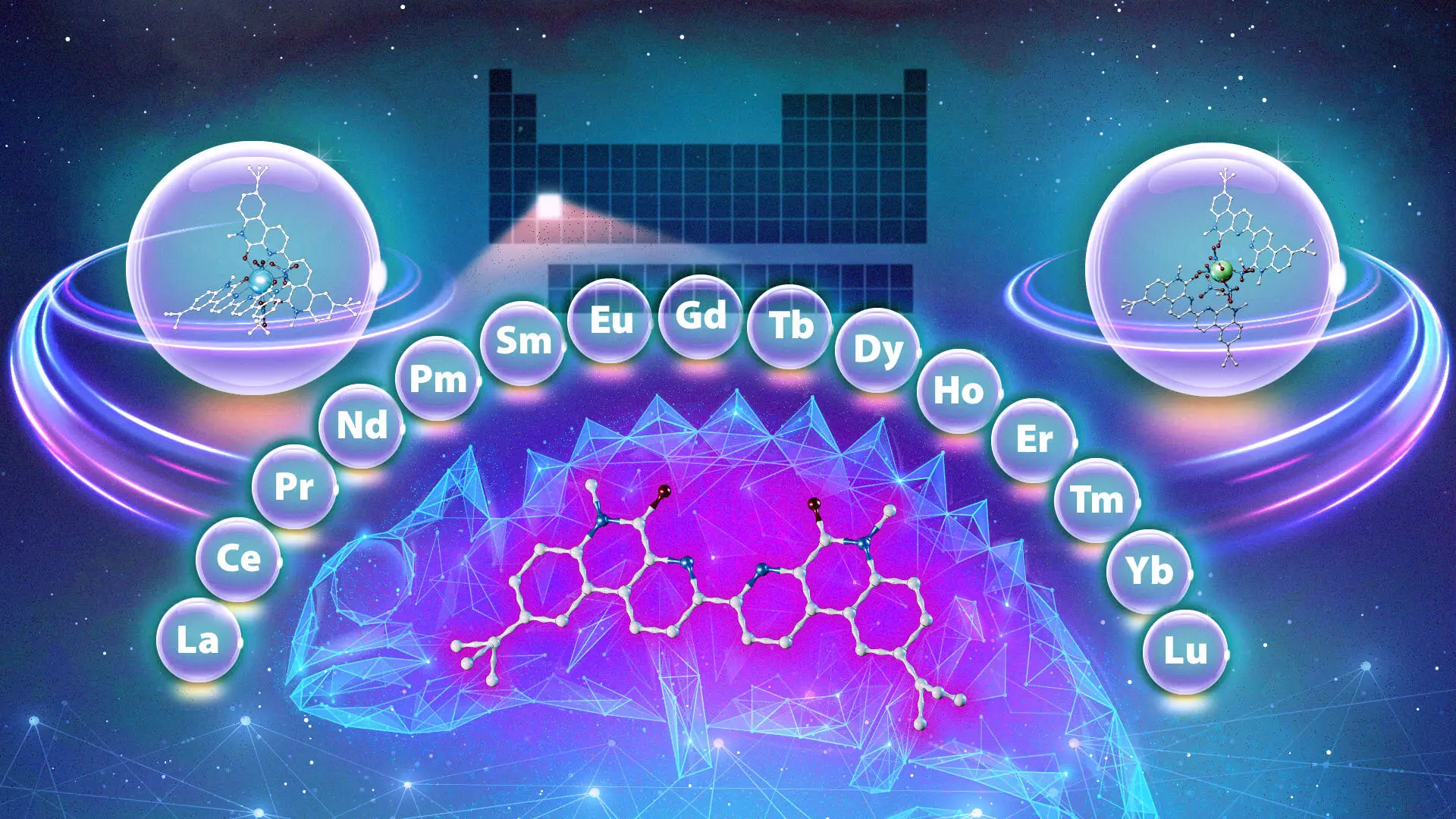
The modern technological landscape, characterized by rapid advancements in clean energy, medical equipment, and national security, increasingly hinges on the efficient use of rare-earth metals. As essential components in various applications—ranging from renewable energy solutions to sophisticated medical imaging systems—the demand for a reliable method to purify these metals has never been greater. The recent research conducted by a team at the Department of Energy’s Oak Ridge National Laboratory (ORNL), in collaboration with Vanderbilt University, sheds light on a revolutionary approach to this challenge. Their discovery of a unique chemical agent, aptly termed the “chameleon ligand,” could dramatically streamline the complex extraction process of lanthanides—elements that are pivotal to various industries but notoriously difficult to isolate.
Often mischaracterized as rare due to their nomenclature, rare-earth metals are, in fact, much more prevalent than their name suggests. The 15 lanthanides, plus two additional elements, exist naturally in mineral ores and are as common as other well-known metals like copper and lead. However, this accessibility is misleading. The inherent challenge arises from the similarity in size and chemical characteristics among these metals, making their individual isolation a formidable task. As highlighted by Subhamay Pramanik, a former ORNL postdoctoral researcher, isolating the unique properties of each lanthanide demands precision in separation science—a task complicated by their almost identical attributes.
Current methods used in the industry for extracting these metals rely on the use of ligands—specialized chemical compounds that selectively bind to specific metals within a solution. This separation process typically involves mixing these ligands with an organic solvent, which does not mix with water. When combined with an aqueous solution containing a mixture of lanthanides, the ligand attempts to capture the desired metal, effectively transferring it into the organic phase. The traditional separation process is generally a multi-step endeavor, requiring extensive time, resources, and often resulting in significant waste that poses environmental challenges.
The breakthrough by the ORNL and Vanderbilt researchers presents a transformative alternative. The ligand they investigated demonstrates remarkable flexibility akin to a chameleon, adjusting its binding behavior according to the unique conditions of its environment. As Santa Jansone-Popova, a co-leader of the study, elucidates, this chameleon ligand can preferentially bind to different lanthanides depending on the acidity of the solution and the duration of interaction. More specifically, under varying conditions, the ligand can target heavier or lighter lanthanides, allowing it to perform multiple separations with a single compound—a significant departure from traditional methods.
What sets this discovery apart is the ligand’s ability to adapt to its surroundings, a versatility not seen in previously utilized ligands. The research indicates that this single compound can effectively separate lanthanides in any order, ranging from the heaviest to the lightest, based on the experimental conditions. This adaptation not only promises to reduce the number of extraction steps required but also holds the potential to minimize overall waste production, thus aligning with more sustainable practices in metal purification.
The implications of this discovery extend beyond immediate industrial applications; it marks a pivotal turning point in our understanding of ligand behavior. The findings suggest that there may be more compounds with similar chameleon-like properties, opening the door for further exploration and development of efficient separation techniques. Researchers can now delve deeper into studying the mechanisms employed by this ligand and pursue the synthesis of new compounds that can offer similarly adaptable behaviors.
The identification of the chameleon ligand presents a significant leap forward in the field of rare-earth metal purification. By redefining the capabilities of ligands, the ORNL research team has not only improved the efficiency of isolating these vital elements but has also introduced a new framework for understanding chemical interactions. As we continue to lean on rare-earth metals in our increasingly tech-driven society, innovations like this will be crucial in fostering sustainable practices and meeting the growing demand for these essential materials while minimizing ecological impact. The future for lanthanide extraction appears more promising than ever, and this breakthrough serves as a testimony to the power of innovative research in overcoming longstanding challenges in the industry.