Innovative strides in catalyst development are essential for optimizing the oxygen evolution reaction (OER), a pivotal aspect of green energy technologies like water splitting and metal-air batteries. Recent research highlights the introduction of chromium (Cr) into transition metal hydroxides as a promising approach to enhance catalytic efficiency. Published in ACS Catalysis on August 30, 2024, these findings underscore the collaboration of theoretical and experimental methodologies to tackle the long-standing challenges in efficiency within electrochemical reactions.
As the world shifts toward sustainable energy solutions, hydrogen production via water electrolysis represents a vital pathway for storing energy from variable renewable sources such as solar and wind. OER serves as one of the most critical half-reactions in this process, yet it has been impeded by its sluggish kinetics. The necessity for high-performance, stable catalysts cannot be overstated, as they can significantly enhance the overall efficiency of hydrogen production systems, thereby contributing to the broader agenda of clean energy adoption.
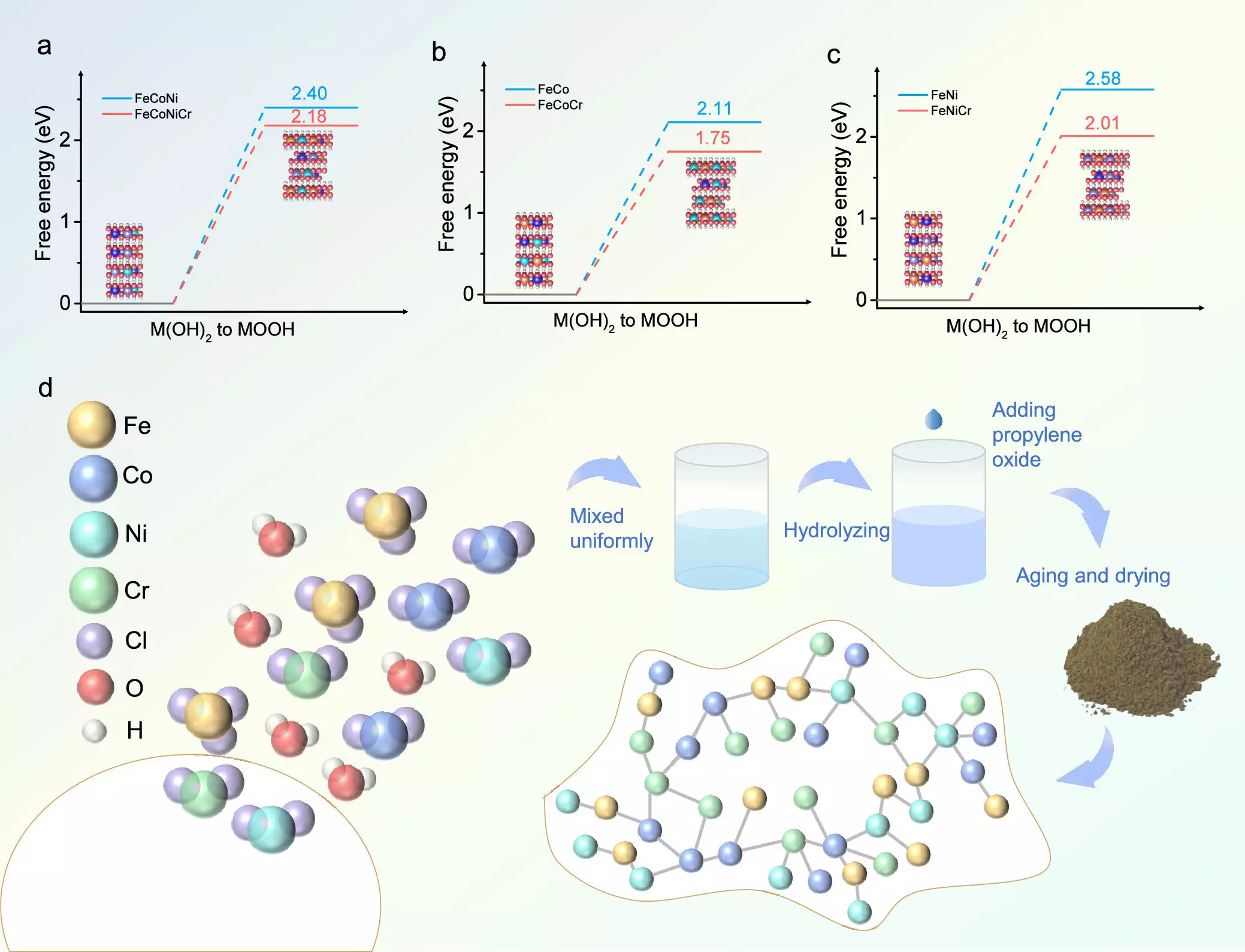
The researchers from Tohoku University’s Advanced Institute for Materials Research (WPI-AIMR) focused on the potential of chromium doping in catalyst performance. By synthesizing a FeCoNiCr hydroxide catalyst through an aqueous sol-gel method, they ensured precise elemental distribution, which contributed to remarkable improvements in catalytic activity. Co-author Hao Li noted that incorporating Cr facilitates a crucial phase transition into a reactive oxyhydroxide state, effectively fast-tracking the OER process.
The synthesized catalyst exhibited an impressive low overpotential of 224 mV in alkaline environments, outperforming existing candidates by a staggering 52 mV. This advancement was complemented by remarkable stability during prolonged testing, showcasing over 150 hours of continuous functionality. Furthermore, a zinc-air battery, utilizing this new catalyst, demonstrated consistent performance over 160 hours with a minimal charging voltage difference of 0.70 V, emphasizing its potential for real-world applications.
The research team employed density functional theory (DFT) calculations to elucidate how chromium doping finely adjusts the electronic properties of active sites, thus enhancing the overall reaction kinetics. The Bader charge analysis revealed that nickel and cobalt retained a stable +3 oxidation state throughout the OER process, a vital condition for sustaining catalytic activity and efficiency throughout extended operation.
Looking ahead, the research team is committed to expanding their investigation into different elements that may offer further catalytic enhancements. Di Zhang, a key co-author, emphasized the innovative methodology that could streamline the discovery of new materials, aiming to develop even more efficient catalysts to support the acceleration of clean energy technologies. With global energy demands increasing, optimizing and fabricating cost-effective catalysts stands as a fundamental requirement for the growth of renewable energy systems.
As researchers continue to refine catalyst technologies, the collaborative efforts of theoretical modeling and practical synthesis are reshaping the landscape of energy solutions. By addressing the catalytic limitations within the OER, this groundbreaking research paves the way for advancements in hydrogen production, thus bolstering the transition towards sustainable and renewable energy infrastructures.