Recent advancements in the study of solvation shells—a crucial aspect of ion behavior in solutions—have emerged from a collaborative research effort involving the Fritz Haber Institute, Sorbonne University, and Uppsala University. The implications of this groundbreaking research, detailed in their paper “The solvation shell probed by resonant intermolecular Coulombic decay,” published in *Nature Communications*, may significantly change how scientists comprehend molecular interactions in various solvents.
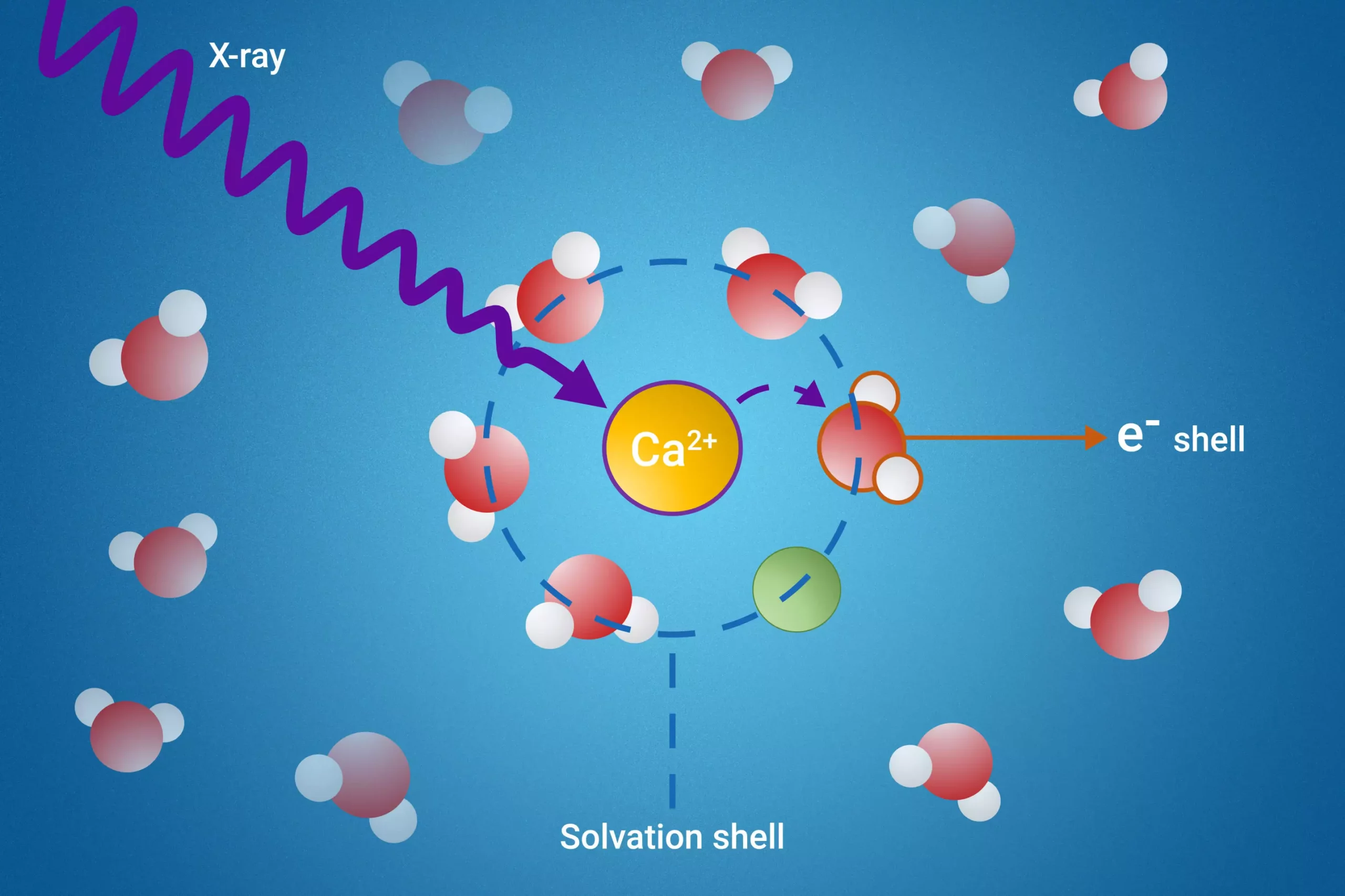
At the heart of this study is the solvation shell, which refers to the structured layer of solvent molecules that encase dissolved ions. This phenomenon is pivotal in various scientific disciplines, as it affects reaction mechanisms, stability, and the physical properties of solutions. Understanding the unique characteristics of these boundary layers can provide insights essential for chemistry, biology, and material science.
However, probing the intricacies of solvation shells has historically been fraught with challenges. The diversity in the properties of solvation shell molecules compared to free solvent molecules complicates experimental studies. Traditional methods lack the precision required to isolate and investigate the specific solvent molecules involved in solvation, often leading to inconclusive results that do not adequately represent the solvation model.
The research team has successfully addressed this long-standing issue by employing a novel technique known as resonant intermolecular Coulombic decay (ICD). This method utilizes the power of X-rays to excite the solvent molecules, allowing researchers to monitor their interactions during the decay process. As the excited states of these molecules transition back to a stable condition, the interactions between solvent molecules reveal detailed information about the solvation shell dynamics.
One of the key revelations of this study is the identification of a specific ICD process that serves as an indicator of ion pair formation. Such insights are critical; they open pathways to better understand not merely how ions interact with their solvents, but also the fundamental mechanisms that govern chemical reactions in various environments. Moreover, the researchers succeeded in measuring the electron binding energies of water molecules present in the first solvation shell—a feat that was previously unattainable and represents a significant leap forward in solvation research.
In a world increasingly reliant on precise measurements and predictive capabilities, these advances are particularly relevant for scientists operating at the intersection of chemistry and technology. From atmospheric science to electrochemistry and beyond, improved understanding of solvation shells can lead to better textural analyses, novel material designs, and enhanced chemical processes.
The innovative methodology introduced by this research team holds promise for various scientific domains, potentially transforming how we approach the study of molecular interactions. With the ability to probe solvation shells with greater accuracy, future research could yield new insights into biochemical processes, advance renewable energy technology, and enhance the efficacy of pharmaceutical compounds.
The collaborative efforts of these esteemed institutions mark a pivotal moment in solvation research. By shedding light on the complex behavior of ions in solutions, the implications of these findings are poised to resonate across numerous scientific fields, encouraging ongoing exploration and discovery that could redefine existing paradigms in chemistry and beyond.