In an exciting development in the field of chemical science, researchers at RIKEN have unveiled a novel approach for harnessing dinitrogen (N2) from the atmosphere to create vital industrial compounds. Published in the prestigious journal Nature, this research points toward a more sustainable and energy-efficient method for synthesizing materials such as polymers and pharmaceuticals. Given the ubiquity of dinitrogen—it constitutes nearly 80% of the Earth’s atmosphere—this breakthrough could signify a paradigm shift in chemical manufacturing processes.
Despite its abundance, the application of dinitrogen in chemical reactions has long been impeded by its strong triple bond, rendering it stable and largely inert. The traditional method for utilizing dinitrogen involves its conversion to ammonia through the Haber–Bosch process, a step that unfortunately demands high energy inputs and intricate procedural steps. Each time a chemical engineer attempts to synthesize useful compounds like alkyl amines from dinitrogen, they must first break the molecule down and react it with activated carbon components, usually derived from alkenes transformed into alcohols or carboxylic acids. This results not only in energy inefficiency but also prolongs the synthesis timeline.
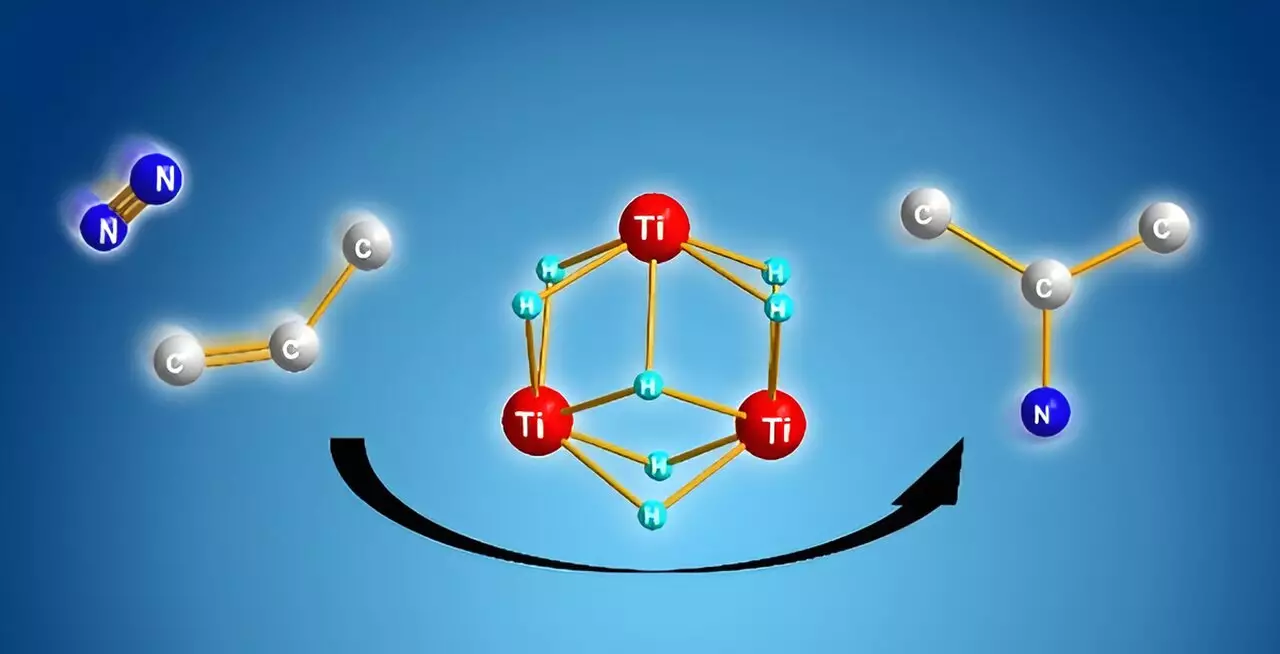
Led by researcher Takanori Shima at the RIKEN Center for Sustainable Resource Science, the team aimed to create a more direct route for chemical reactions, eliminating unnecessary and energy-consuming steps. Their breakthrough came through the utilization of titanium polyhydrides—complexes involving titanium atoms interconnected by hydrogen. This innovative use of titanium polyhydride generated significant reactivity with stable small molecules such as dinitrogen and benzene. For the first time, it became feasible to transform dinitrogen directly into useful alkyl amines through a cooperative interaction facilitated by titanium-hydride units.
In their experimental setup, Shima and his team established that when they combined alkenes with titanium polyhydride, the alkenes underwent activation while leaving several titanium–hydride units intact. Upon introducing dinitrogen into the mixture, these free titanium–hydride units enabled the cleavage of the dinitrogen molecule. This was followed by the formation of a novel nitrogen–carbon bond between the activated nitrogen and the carbon species, ultimately yielding alkyl amines. The findings from computational models illuminated the high likelihood of nitrogen–carbon bond formation over other possible bond formations, corroborating the efficiency of this new reaction pathway.
The potential applications of this discovery are vast. By simplifying the synthesis of nitrogen-containing compounds and reducing energy consumption, industries could significantly lower their environmental footprint while also decreasing production costs. The creation of alkyl amines—key intermediates used in the synthesis of various pharmaceuticals—could be revolutionized, making essential medications more accessible. As the world grapples with climate change and the need for sustainable practices, such advances in chemical synthesis are both timely and necessary.
Looking ahead, Shima and his research group are investigating further developments to transform this process into a catalytic system. The goal is to create a continuous and efficient method that could be readily integrated into existing industrial platforms. With the ability to directly utilize dinitrogen and alkenes under mild conditions, this research not only paves the way for energy-efficient synthesis but also sets the stage for innovations in sustainable chemistry that could have far-reaching consequences.
The exploration of dinitrogen’s potential marks a significant advancement in the quest for sustainable chemical processes. By enabling direct reactions that convert abundant atmospheric nitrogen into valuable compounds, researchers are moving closer to creating more eco-friendly industrial practices. As investigations continue and the applications of this discovery are realized, the future of chemical synthesis holds tremendous promise for sustainability and efficiency. This remarkable advancement showcases how fundamental scientific research can have profound implications for industries and the environment alike.