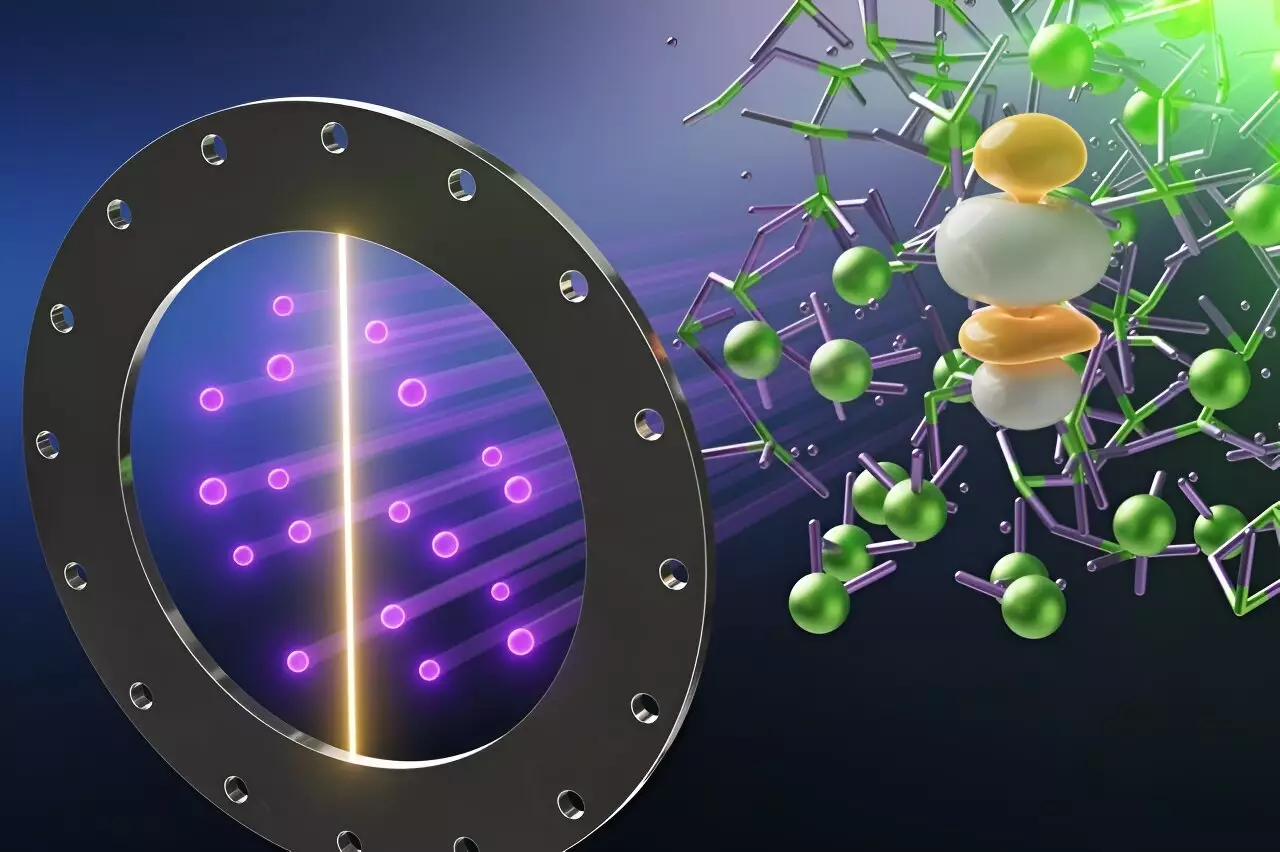
In a recent groundbreaking study published in the *Journal of the American Chemical Society*, scientists have unveiled important insights into the chemistry and structural dynamics of high-temperature liquid uranium trichloride (UCl3) salt. This exploration represents a critical advancement in our understanding of nuclear fuel sources, particularly for next-generation reactors. Conducted by researchers at Oak Ridge National Laboratory (ORNL), in collaboration with Argonne National Laboratory and the University of South Carolina, this study is poised to pave the way for enhanced predictive models essential for reactor design.
Molten salt reactors have gained renewed attention as a promising solution for generating safe and affordable nuclear energy. The frameworks to build such reactors have historical roots tracing back to ORNL’s prototyping experiments in the 1960s, which successfully laid the groundwork for this technology. Today, as the global community intensifies its focus on decarbonization, various nations are rekindling their ambitions to implement these advanced nuclear reactors widely.
To optimize the design of future molten salt reactors, a profound understanding of the behavior of liquid fuel salts is fundamental. This contrasts starkly with conventional nuclear reactors that typically utilize solid uranium dioxide pellets. The unique behaviors of these fuel salts, especially at the atomic level, pose significant challenges for researchers, primarily due to the complexities brought on by the radioactive nature of actinides like uranium.
The team utilized a combination of computational methods alongside the advanced capabilities of the Spallation Neutron Source (SNS) located at ORNL. This facility, known for its exceptional neutron scattering techniques, enables researchers to observe atomic interactions, bonds, and dynamic behavior in various materials. By directing neutron beams at samples of liquid UCl3, the scientists gleaned critical insights into the positioning, mobility, and magnetic characteristics of the material’s atoms.
The neutron scattering process is akin to a game of billiards, where neutrons interact with atomic nuclei, scattering off at varying angles. By detecting and analyzing these scattered neutrons, researchers can construct a detailed map of atomic arrangements and interactions. However, conducting such experiments at extreme temperatures—as high as 900 degrees Celsius—merely adds to the complexity, necessitating stringent safety measures.
One of the most astonishing discoveries from this research was the counterintuitive observation that as UCl3 transitions to a liquid state, the average distance between the uranium and chlorine bonds actually decreases. This phenomenon contradicts the general principle in chemistry where heating typically leads to bond expansion due to increased molecular movement. Instead, the team noted varying bonding lengths oscillating rapidly, revealing a potentially new aspect of atomic interaction dynamics that had previously gone unnoticed.
Alex Ivanov, a co-leader of this study, emphasized the preeminence of these findings in the field of actinides. The research indicates that the characteristics of UCl3 in its molten state, which oscillate from short to long bond lengths at astonishing speeds, represent a departure from conventionally understood chemical behavior.
The revelations from this study have far-reaching implications. For instance, the observation of transient covalent bonding characteristics within the otherwise predominantly ionic framework of UCl3 at high temperatures complicates previous models of molten salt chemistry and challenges established norms in the field. Understanding the fundamental atomic interactions within actinides under these extreme conditions can also aid in addressing contemporary issues surrounding nuclear waste management and pyroprocessing techniques.
These findings may thus enhance both experimental methodologies and computational approaches, facilitating more effective designs for upcoming nuclear reactors and advancing the broader agenda of sustainable nuclear energy. The study contributes not just to the chemistry of actinides but also positions these researchers, and future scientists, at the forefront of tackling challenges associated with energy production in a warming world.
While the journey of understanding the intricate behaviors of liquid uranium trichloride is just commencing, this significant study marks a pivotal step forward. With deeper insights into the molecular dynamics of actinides at high temperatures, researchers are now better equipped to pursue innovative reactor designs that promise safer and more efficient nuclear energy solutions. As the quest for effective decarbonization continues, advancements in nuclear technology could profoundly reshape the energy landscape of the future.