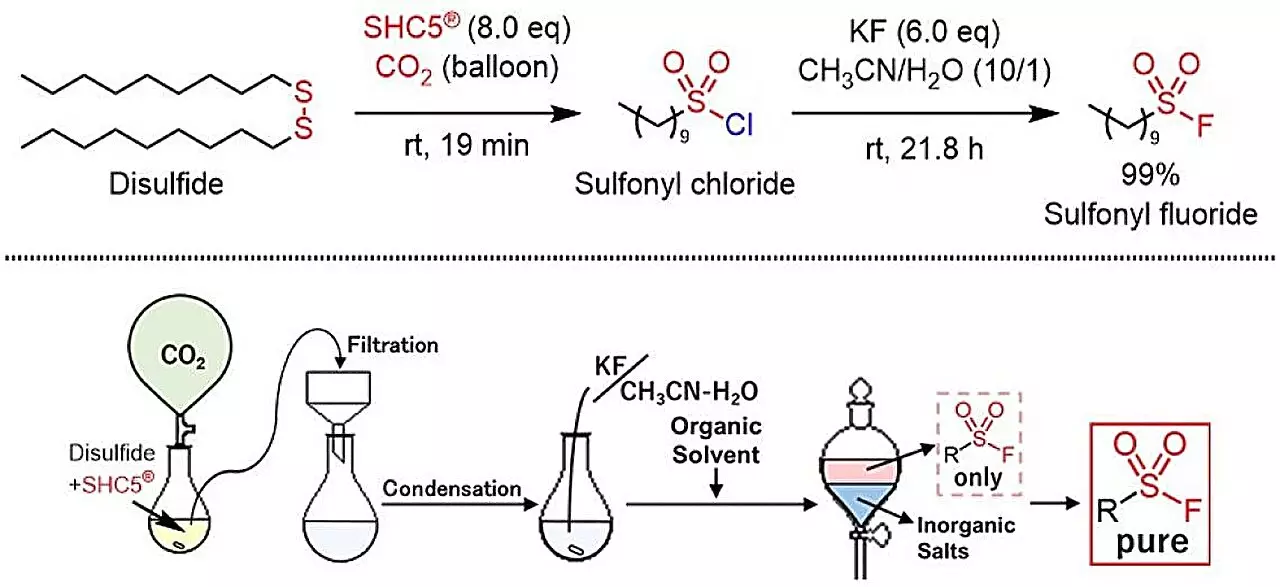
In recent years, the field of chemistry has witnessed a paradigm shift towards more sustainable practices, aiming to align with global environmental goals. A significant development in this movement is the recent synthesis of sulfonyl fluorides from thiols and disulfides, utilizing SHC5 and potassium fluoride (KF). This innovative method introduces a highly efficient and environmentally friendly alternative, moving away from conventional synthetic processes that often produce hazardous byproducts. The culminating research, published in ACS Sustainable Chemistry & Engineering, heralds a new era for click chemistry and its applications in various scientific disciplines.
Click chemistry, a concept that gained traction in the early 2000s, promotes the rapid and efficient assembly of molecular building blocks with minimal byproducts. Characterized by its high selectivity and exceptional yields, click chemistry has found applications across fields ranging from materials science to pharmaceutical development. Nevertheless, the synthesis of sulfonyl fluorides—a crucial element in sulfur-fluorine exchange reactions—has historically relied on toxic and cumbersome reagents, such as SO2F2 gas. This limitation has presented a significant barrier to researchers seeking to incorporate sulfonyl fluorides into their work.
The newly developed method represents a significant breakthrough in the synthesis of sulfonyl fluoride compounds. By utilizing the relatively benign reagents SHC5 and KF, researchers are able to achieve the desired sulfonyl fluoride synthesis safely and effectively. This green approach stands out not only for its efficiency but also for its favorable environmental profile, producing sodium chloride (NaCl) and potassium chloride (KCl) as the sole byproducts—both of which are non-toxic and easily managed.
The implications of this process extend far beyond mere chemical curiosity. The ability to synthesize a diverse range of sulfonyl fluoride compounds, including those with aromatic and heterocyclic components, opens new avenues for research and application. The simplicity of the protocol ensures that the methodology is not only cost-effective but also inherently scalable for industrial application.
As the chemical and industrial sectors increasingly prioritize sustainability, this new method for synthesizing sulfonyl fluorides is poised to become a cornerstone. The minimal environmental impact and ease of production make it an attractive alternative for industries that rely heavily on sulfur-fluorine chemistry. Moreover, the focus on reducing hazardous waste aligns seamlessly with the objectives outlined in the Sustainable Development Goals (SDGs), highlighting a commitment to not only innovation but also responsibility.
The corresponding authors of the study, Masayuki Kirihara, Shinobu Takizawa, and Mohamed S. H. Salem, emphasize the necessity of developing chemical processes that consider environmental ramifications. This research aligns with a growing trend among chemists to prioritize sustainable methodologies that contribute positively to both scientific advancement and environmental stewardship.
The synthesis of sulfonyl fluorides through a safe, efficient, and environmentally friendly process marks a significant step forward in the field of sustainable chemistry. By integrating concepts of green chemistry into synthetic practices, researchers are not only advancing chemical knowledge but also paving the way for responsible industrial applications. As the global community continues to grapple with environmental challenges, innovations such as this one may help forge a path towards a cleaner, more sustainable future in chemical production.