The ever-evolving field of atmospheric chemistry continues to reveal new and intricate details about the nature of our atmosphere. Recently, a groundbreaking discovery from the Leibniz Institute for Tropospheric Research (TROPOS) in Leipzig has brought attention to sulfurous acid (H2SO3), a compound that had long been deemed elusive. For the first time, scientists have successfully demonstrated the presence of sulfurous acid in the gas phase under atmospheric conditions. This pivotal finding, published in the renowned journal Angewandte Chemie, highlights the dynamic processes occurring in our atmosphere and challenges long-standing assumptions regarding sulfur compounds.
Sulfurous acid has historically been overshadowed by its more familiar counterpart, sulfuric acid (H2SO4). Textbooks often suggest that H2SO3 could form in aqueous solutions of sulfur dioxide (SO2), but isolated detection was assumed impossible. Despite numerous attempts to observe H2SO3 using various spectroscopic techniques, researchers could only identify its progeny: bisulfite (HSO3−) and sulfite (SO3^2−). The only preceding evidence of H2SO3’s existence was obtained in 1988 by Helmut Schwarz’s team at the Technical University of Berlin, which involved generating the compound in an in-situ setup within a mass spectrometer. Their findings suggested that H2SO3 had a fleeting lifespan of around 10 microseconds in a vacuum, thereby complicating its study.
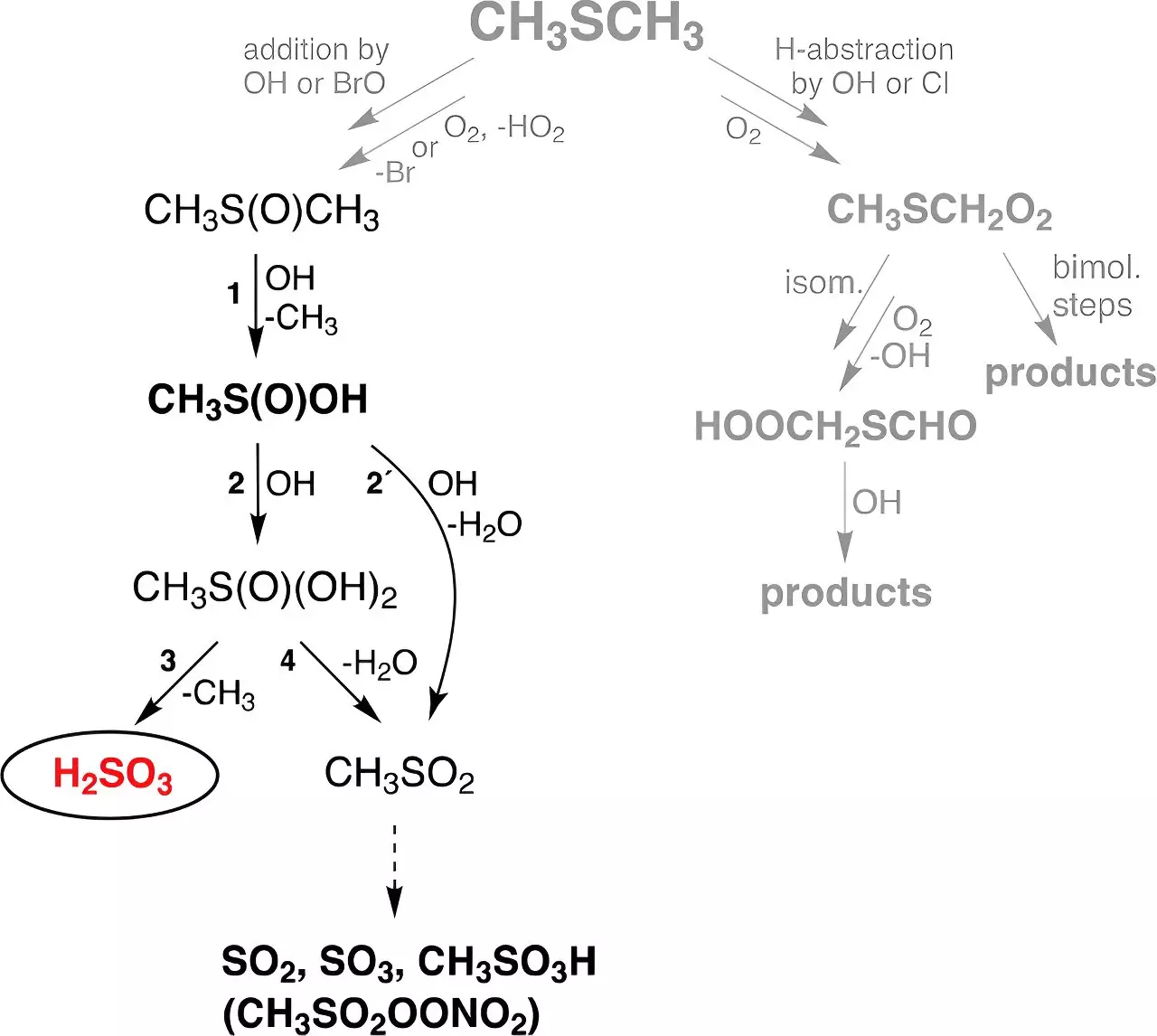
Theoretically, the formation of H2SO3 was indicated as a product of gas-phase reactions between hydroxyl (OH) radicals—emanating primarily from ozone and water vapor under ultraviolet radiation—and dimethyl sulfide (DMS). DMS is a substantial biogenic source of sulfur, significantly derived from marine biological processes, contributing about 30 million tonnes to the atmosphere annually. This suggests that DMS is a crucial player in atmospheric chemical dynamics.
The researchers at TROPOS undertook an experimental campaign to investigate the plausible pathway leading to H2SO3 formation from DMS. Their findings were striking; they established a clear and observable signature of H2SO3 in gas-phase reactions under simulated atmospheric conditions. Notably, sulfurous acid exhibited remarkable stability for approximately thirty seconds, regardless of humidity levels, although longer observation periods remain to be examined.
Dr. Torsten Berndt, who spearheaded the project, expressed his astonishment at the definitive detection of H2SO3, a compound previously thought to be merely theoretical. The implications of this discovery are profound, leading to the integration of newly acquired data into a global chemistry-climate model.
Simulations stemming from the TROPOS findings indicated that sulfurous acid could be formulated in significant quantities—a staggering 8 million tons globally each year. Remarkably, the yield of H2SO3 was estimated to exceed theoretical predictions, producing approximately 200 times more mass than the direct conversion of DMS into sulfuric acid. This revelation enhances our understanding of the already complex sulfur cycle in the atmosphere and may refine our models of atmospheric chemistry.
Despite these breakthroughs, questions linger. While sulfurous acid displays some stability in the gas phase, its interaction with trace gases in the atmosphere and its reaction with water vapor remains inadequately understood. Dr. Berndt emphasized the necessity for additional research to delineate the role and impact of H2SO3 in atmospheric processes further.
This new avenue of investigation is emblematic of the continual evolution of atmospheric science, showcasing the importance of methodical experimental work combined with advanced detection techniques. The use of high-sensitivity mass spectrometry, which can identify products at concentrations as low as 10^4 molecules per cubic centimeter—amidst a backdrop of 10^15 molecules—demonstrates the potential for future breakthroughs in identifying and understanding atmospheric compounds.
As atmospheric chemistry advances, the possibilities for revealing new reaction pathways and validating theoretically proposed compounds expand. The findings on sulfurous acid not only enrich our body of knowledge regarding atmospheric processes but also underscore the importance of innovative methodologies in chemical research. The exploration of H2SO3 may indeed be just the beginning, as scientists delve deeper into the myriad complexities of our atmosphere and its chemical behaviours.