Fuel cells represent a significant advancement in energy conversion technologies, allowing for the generation of electricity through electrochemical processes rather than combustion. This method offers a clean alternative, reducing air pollution significantly in comparison to traditional energy sources. Their applications stretch across a variety of technologies, including electric vehicles, portable electronic devices, and industrial power systems. However, the widespread implementation of fuel cells has been hindered by the reliance on expensive materials, particularly precious metal catalysts, which drives up production costs.
The challenge posed by high-cost materials is a significant barrier to the commercialization of fuel cells. Precious metals like platinum are often used as catalysts due to their efficiency; however, their scarcity and high market price create an economic hurdle. In response to this, researchers in recent years have focused on developing Anion-Exchange Membrane Fuel Cells (AEMFCs), which utilize more abundant and cost-effective catalysts. While these fuel cells are promising, a notable issue remains: many non-precious metal catalysts suffer from self-oxidation, jeopardizing their operational stability and longevity. This dilemma has sparked extensive research and innovation aimed at enhancing the resilience of these alternative catalysts.
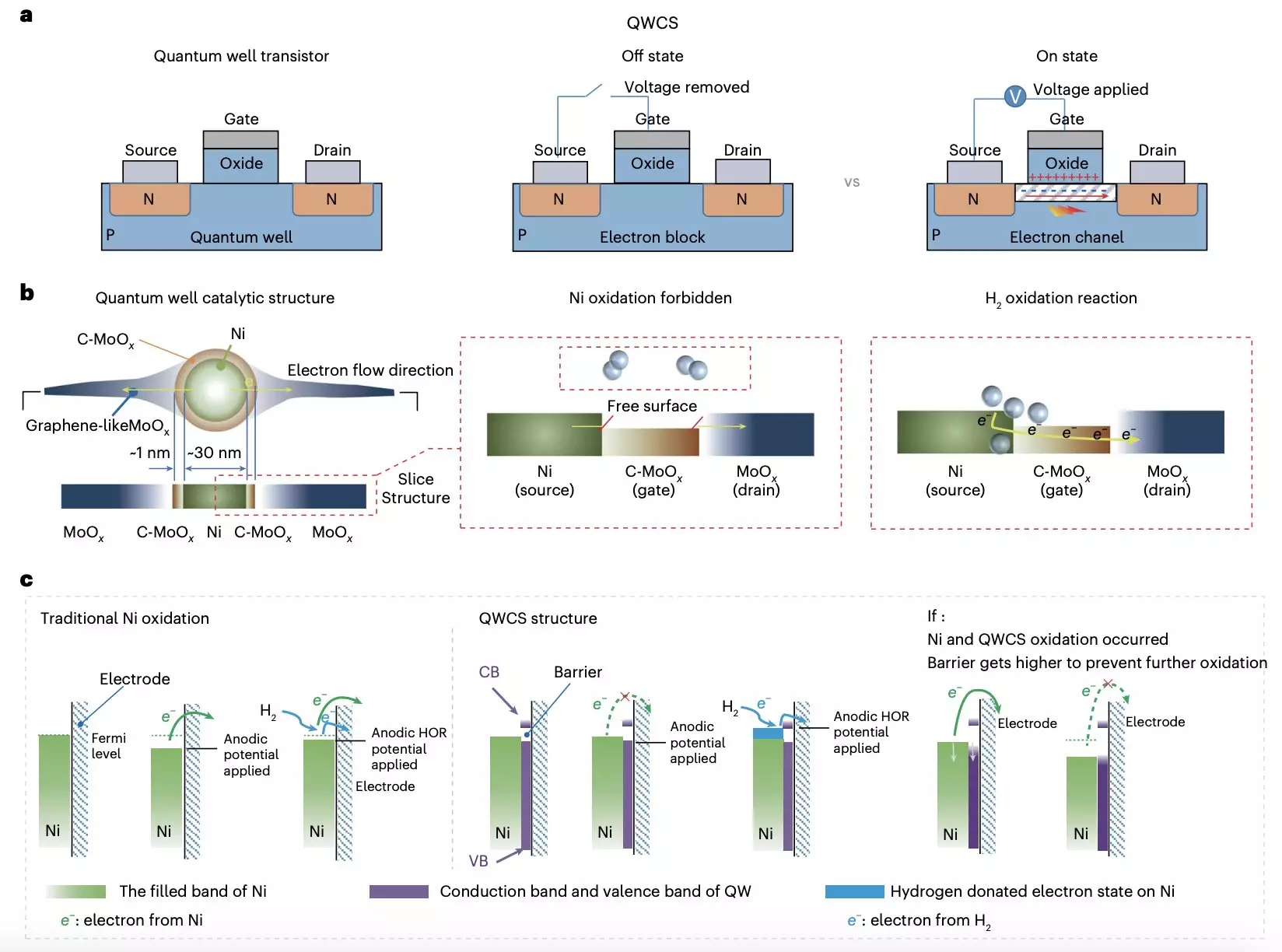
Recent contributions from teams at Chongqing University and Loughborough University have marked a significant leap in addressing the self-oxidation challenge faced by non-precious metal catalysts. Their innovative approach involves the use of a novel quantum well-like catalytic structure (QWCS) that is designed to enhance the performance and durability of metallic nickel electrocatalysts in AEMFCs. This design utilizes quantum-confined nickel nanoparticles embedded within a composite heterojunction, creating a robust environment that mitigates the likelihood of electro-oxidation.
In a pivotal study published in Nature Energy, the research team elaborated on the QWCS framework. The incorporation of carbon-doped molybdenum oxide (C-MoOx) serves as a low-energy valley while amorphous MoOx acts as a high-energy barrier. This arrangement not only enhances the catalytic ability but also ensures that electrons released during the hydrogen oxidation reaction are selectively transferred, thus protecting the catalyst from degradation processes typically associated with self-oxidation.
Operational Efficacy and Mechanical Resilience
The performance metrics of this newly conceived Ni@C-MoOx catalyst are compelling. Under rigorous operational conditions, the catalyst demonstrated exceptional stability, maintaining its performance metrics during long-term testing over a span of 100 hours. More impressively, the developed fuel cell achieved a remarkable power density of 486 mW mgNi⁻¹ while exhibiting no degradation in performance even after undergoing multiple shutdown-start cycles—an endeavor where traditional counterparts without the QWCS would typically falter.
This combination of operational performance and mechanical resilience highlights the immense potential of QWCS technology in revitalizing the field of fuel cells. The researchers noted that the electrons within the nickel nanoparticles experienced a stabilization barrier, which safeguarded them from electro-oxidation while allowing electrons generated from the hydrogen oxidation reaction to pass freely. This innovation could ultimately reshape the landscape of fuel cell technology by providing a pathway toward economically viable and reliable systems.
The advancements in QWCS structures hold promising implications for the future of fuel cells. As the world increasingly pivots toward sustainable energy solutions, the development of cost-effective AEMFCs with enhanced durability could facilitate broader adoption across various sectors. Moreover, the innovative strategies underpinning this research can foster the next generation of catalysts that leverage quantum confinement principles, paving the way for further breakthroughs.
The exploration and enhancement of non-precious metal catalysts, particularly through the innovative design of quantum well-like structures, represent a crucial stride toward the mainstream adoption of fuel cells. By overcoming existing barriers to stability and performance, researchers are not only contributing to cleaner energy technologies but also setting the stage for a sustainable future powered by renewable energy sources.