The path to technological advancements has often been paved by relentless experimentation. One notable historical figure, Thomas Edison, famously tested thousands of materials before successfully creating the incandescent lightbulb with tungsten filament. This foundational approach has spurred iterative innovation across various fields, laying the groundwork for countless inventions that enhance our daily lives. Today, as we face increasingly complex challenges, the principles of trial-and-error remain crucial, especially in the realm of energy storage and battery systems.
In developments regarding energy storage, it is imperative to understand that the interplay between materials and their functional design is critical. Modern engineers are charged not only with experimentation but also with a thorough comprehension of the physical principles governing material behaviors. This knowledge is essential for crafting advanced materials that meet the demanding requirements of contemporary technology.
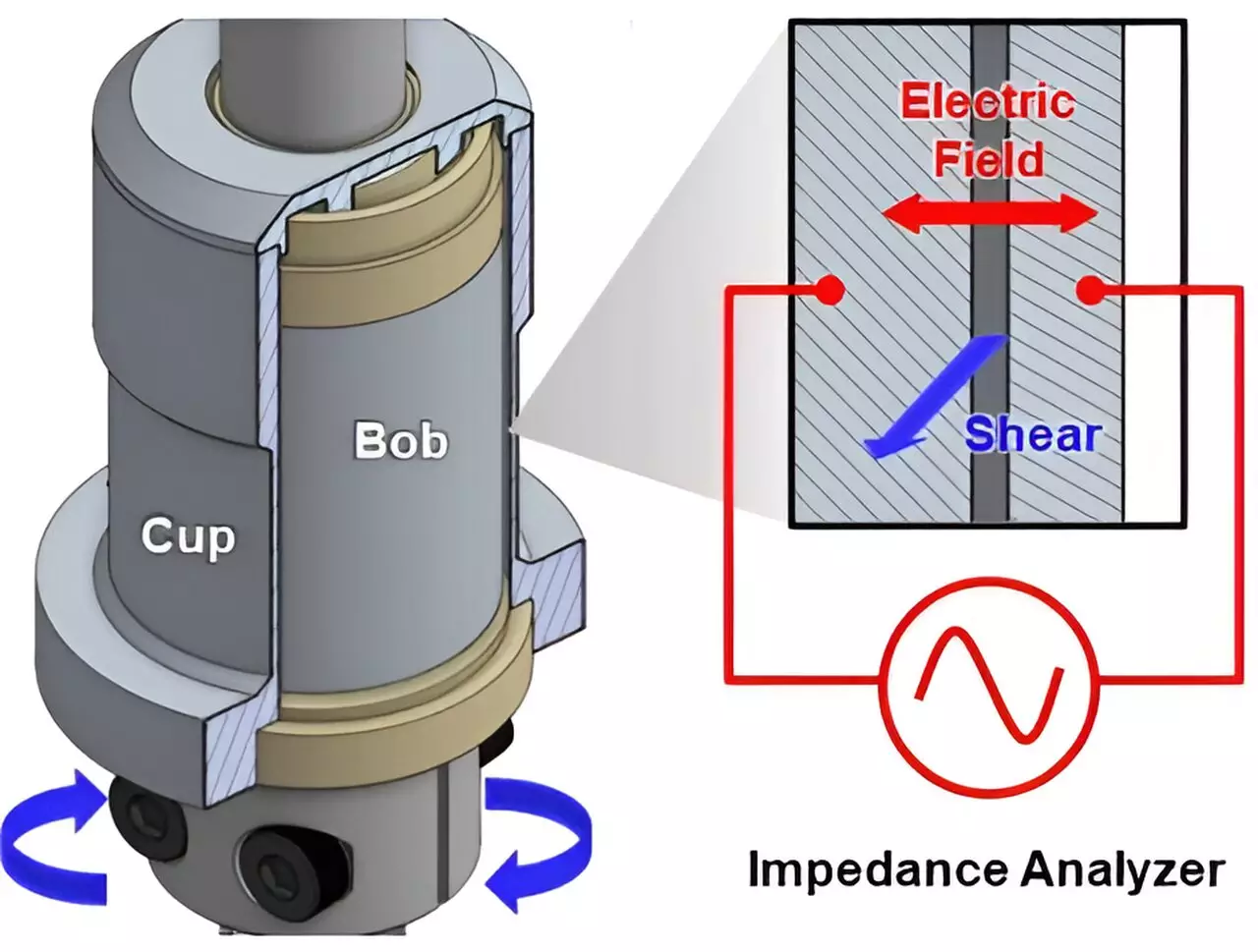
A recent study published in the *Proceedings of the National Academy of Sciences* has shed light on a specific aspect of electrochemical devices: the movement of electrons in conductive slurries used in batteries. Researchers from the University of Delaware (UD) and Northwestern University, along with industry partners, focused on tackling the challenges posed by the conductive components within these slurries. Their collaborative work highlights a significant knowledge gap regarding how electrons transition across conductive particles, vital in enhancing battery efficiency.
Lead researchers included prominent figures such as Norman Wagner from UD and Jeffrey Richards from Northwestern University, both of whom have focused their efforts on examining the dynamics of electrons in these complex materials. The research team’s approach encapsulates a balance between meticulous experimental design, cutting-edge theoretical insights, and advanced simulations.
According to Wagner, achieving ideal performance in electrochemical devices necessitates attention not only to chemistry but also to the microstructures of the materials involved. The final arrangement of components in a slurry dictates the pathways through which electrons can traverse, a factor that directly influences the device’s power output and efficiency. The findings assert that merely focusing on the chemical makeup of the materials isn’t sufficient; one must also consider how these materials interact at a microstructural level during processing and manufacturing.
To illustrate this concept, one could liken the complexities of energy storage systems to a diverse set of race cars on a racetrack. While all vehicles share common components, such as engines and wheels, variations in their design and assembly can result in disparate performances. Similarly, in electrochemical devices, the arrangement and combination of carefully selected materials can drastically alter their efficiency.
A focal point in the study is carbon black, a conductive material widely utilized in batteries and electrochemical devices. Composed of nano-sized carbon aggregates, carbon black plays a pivotal role in the conductivity of slurries. However, electron movement within slurries is not as straightforward as one might assume. While electrons can swiftly navigate through the carbon black aggregates, they must also “hop” from one cluster to another, introducing a layer of complexity due to the nature of the slurry.
This prior research has revealed the critical impact of rheology, or the flow properties, of carbon black on performance. By leveraging neutron scattering techniques, researchers developed a deeper understanding of how the flow dynamics influence conductance within the slurries.
Through their extensive study, the research team has outlined a universal roadmap for understanding the relationship between slurry conductivity and the chemistries involved. This pivotal guideline serves not only as a resource for current systems but also as a foundation for future innovations in electrochemical devices. By gaining insights into how the structural makeup of carbon black slurries affects performance, engineers can better predict and manipulate material behaviors during device manufacturing.
This foundational research can potentially extend to various applications beyond battery technology, including electrolyzers and water deionization technologies. For instance, the challenges associated with splitting water into hydrogen and oxygen can be mitigated by refining the properties of material solutions—an aspect that relates directly to the researchers’ findings.
As stated by Wagner, effective outcomes depend heavily on the processing of materials. It is not enough to have the right chemical composition; without precise manufacturing techniques, the desired performance remains out of reach. The study emphasizes the necessity of integrating knowledge of chemical interactions with an understanding of material processing, ensuring that innovations in energy storage technologies are both efficient and effective.
As we delve deeper into the realm of electrochemical devices, embracing a collaborative spirit that melds rigorous experimentation with a profound understanding of material science will be paramount. Only through such multifaceted approaches can we expect to tackle the energy challenges of tomorrow. The implications of this research are both extensive and exciting, paving the way for breakthroughs that could redefine how we store and utilize energy.