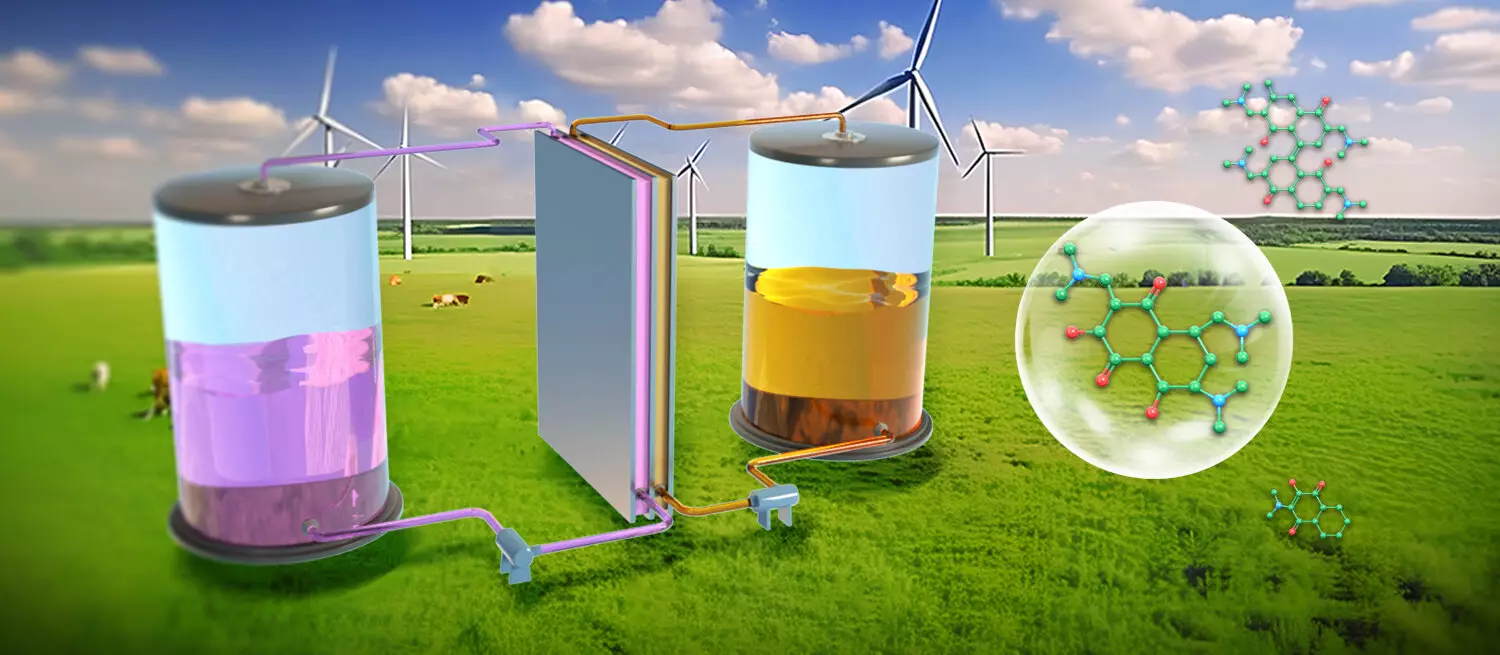
Organic redox-active molecules (ORAMs) have emerged as vital components in the quest for sustainable and efficient energy storage solutions, particularly within the realm of aqueous organic flow batteries (AOFBs). Their structural diversity offers a wealth of possibilities for designing low-cost energy systems. Despite this potential, the inherent challenge of maintaining the stability of these molecules during the operational cycles of charge and discharge is significant. Instability can result in unwanted side reactions, leading to deactivation of the ORAMs and diminishing their functional performance.
Air stability is a prominent concern when working with ORAMs. Many traditional molecules are susceptible to oxidative and hydrolytic degradation when exposed to atmospheric conditions, complicating their practical application. This presents a barrier to the long-term deployment of AOFB technology, as the molecules may require additional protective measures, which could undercut their cost-effectiveness. Addressing these challenges is imperative, as irreversible capacity loss could severely shorten the lifespan of energy storage systems.
A groundbreaking study led by Prof. Li Xianfeng and Prof. Zhang Changkun from the Dalian Institute of Chemical Physics has made significant strides in overcoming these challenges. The researchers synthesized novel naphthalene derivatives featuring active hydroxyl and dimethylamine functionalities that demonstrated remarkable stability in air, functioning effectively as catholytes in AOFBs. The study, published in *Nature Sustainability*, reveals that these naphthalene derivatives are capable of sustaining long-term cycling stability, even in the presence of air—an essential trait for practical applications.
Utilizing a combination of chemical synthesis and in situ electrochemical methods, the research team streamlined the production of these active naphthalene derivatives. This innovation not only simplified the purification process but also contributed significantly to the reduction of overall synthesis costs. The naphthalene derivatives exhibited structural modifications during electrochemical cycling that accentuated their stability, with a multisubstituted architecture that enhances solubility in water while mitigating the risk of side reactions.
The performance of the naphthalene-based AOFB is noteworthy, showcasing an impressive 850 cycles over approximately 40 days at a capacity of 50 Ah L-1. Even in continuous air flow conditions, the batteries maintained stable operations for around 600 cycles with negligible capacity loss, solidifying the air stability of the novel catholytes.
Importantly, the researchers scaled the production of the naphthalene derivatives to the kilogram scale, achieving efficiencies that support industrial applications. Pilot-scale battery systems exhibited a significant system capacity of approximately 330 Ah, coupled with extraordinary cycling stability—maintaining 99.95% capacity retention over 270 cycles.
Prof. Li’s optimism reflects the potential impact of these findings on the future of energy storage technologies. This new class of air-stable ORAMs opens avenues for the development of sustainable energy systems that operate efficiently without the need for inert gas environments, thereby promoting both ecological and economic sustainability in energy storage solutions.
The advancements demonstrated by the Dalian Institute researchers not only contribute to the practical viability of AOFBs but also pave the way for innovative designs in air-stable electrochemical energy storage technologies.