Modern industries and laboratories continually face the critical challenge of synthesizing organic molecules efficiently and safely. The traditional approach primarily revolves around conducting chemical reactions in liquid phases, which facilitates interaction among substrates. However, this method is not without its complications. Many crucial substrates and catalysts exhibit sensitivity to water, leading to undesirable side reactions that produce unwanted byproducts. Consequently, synthetic chemists often resort to utilizing toxic organic solvents. Alarmingly, over 80% of the waste generated from chemical processes arises from these solvents, and inadequate disposal methods heighten environmental concerns.
The situation calls for innovative strategies that can reduce reliance on harmful solvents while maintaining the efficacy of chemical reactions. This need has sparked investigations into alternative catalytic methods and sustainable materials that can revolutionize the processes involved in organic synthesis.
A Breakthrough in Surfactant Development
A team of researchers from the Department of Inorganic and Physical Chemistry at the Indian Institute of Science (IISc) has presented a promising solution to these challenges through their recent study published in ACS Sustainable Chemistry & Engineering. The researchers have adeptly synthesized a surfactant from agricultural waste, specifically derived from cashew nut shell liquid (CNSL). This waste product has minimal economic burdens since India, the world’s second-largest cashew producer, provides ample availability.
The surfactant, named CNSL-1000-M, is indicative of a growing trend in green chemistry where the focus is not solely on reducing harmful chemicals but also on utilizing renewable resources. Pritesh Keshari, the first author of the study, emphasizes that the choice of a bio-based alternative to organic solvents was pivotal in their research approach. This sustainable material is created by merging cardanol—an oily hydrophobic compound found in CNSL—with m-PEG, a hydrophilic polymer. The resulting surfactant showcases the potential to facilitate reactions in an aqueous environment without compromising the sensitivity of the catalysts involved.
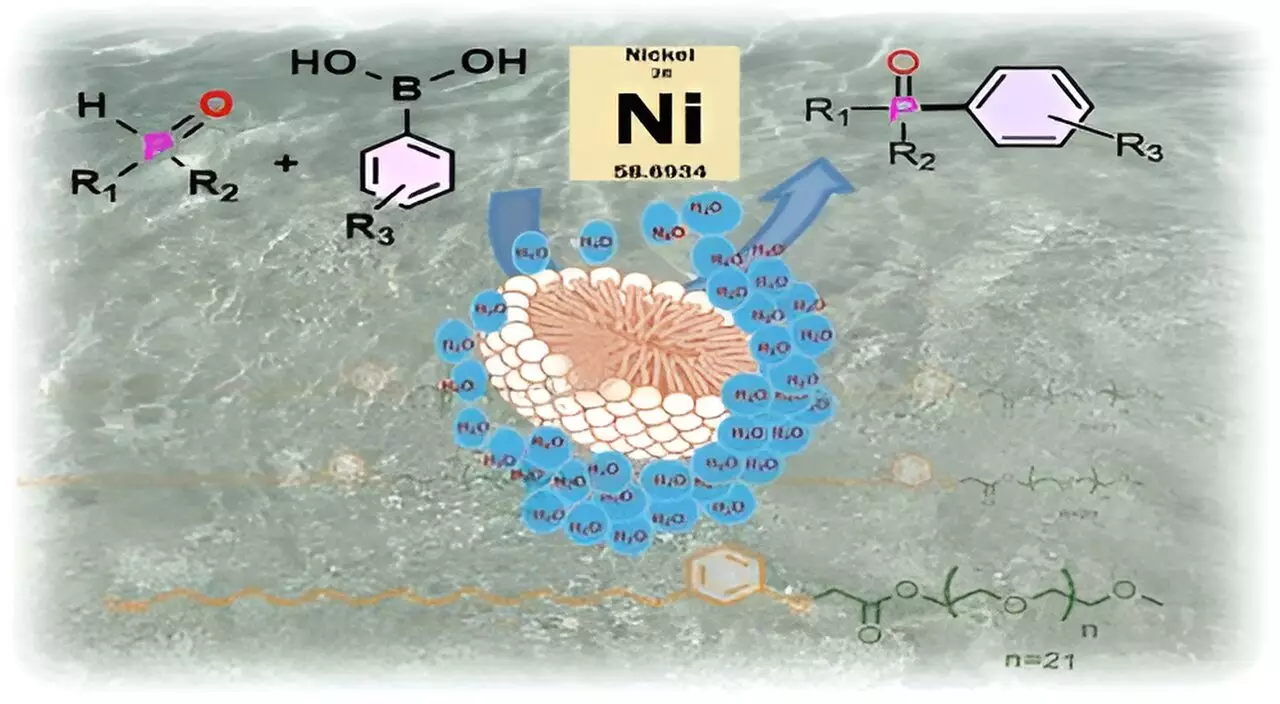
Unique to this surfactant is its ability to form micelles in water. When CNSL-1000-M is introduced into an aqueous solution, the surfactant molecules organize themselves into tiny spherical structures. The internal hydrophobic center of these micelles creates sanctuaries for sensitive substrates and catalysts, effectively shielding them from water. This concept parallels natural biological systems where enzymes utilize hydrophobic pockets to execute critical reactions.
Assistant Professor Susanta Hazra draws an analogy of a football adrift in water; as long as the ball’s surface remains intact, it keeps water at bay. Similarly, substrates housed within micelles are insulated from the surrounding water, providing a controlled environment conducive to chemical reactions. This effective isolation offers a stark contrast to conventional liquid-phase synthesis, where unwanted side reactions frequently occur.
The researchers highlighted the practical implications of their work by demonstrating the use of CNSL-1000-M in catalyzing the formation of carbon-phosphorus bonds, essential in creating diverse compounds, including anticancer drugs like Brigatinib and advanced organic LEDs. The results were compelling: the innovative surfactant produced yields up to 80% higher in water compared to traditional organic solvents. Additionally, the performance of CNSL-1000-M surpassed existing synthetic surfactants by yielding 30% more product in aqueous conditions.
These promising metrics suggest that CNSL-1000-M is not only an environmentally conscious alternative but also economical. The possibility of substituting expensive palladium catalysts with more affordable nickel complexes presents an additional financial incentive for industries. Furthermore, facilitating reactions at lower temperatures makes this surfactant a viable candidate for a wide array of industrial applications.
The researchers express an eagerness to collaborate with industrial partners to implement this aqueous micellar technology on a larger scale. The ambition extends beyond just validating their findings; they aim to refine micellar chemistry further, paving the way for broader adoption of sustainable practices within the chemical industry.
The development of CNSL-1000-M serves as a turning point in the quest for greener chemical synthesis. By harnessing agricultural waste to create an effective surfactant that mitigates reliance on hazardous solvents, researchers at IISc have made significant strides in addressing both environmental and economic challenges in organic chemistry. The ongoing exploration into micellar catalysis not only exemplifies innovation but also sets the stage for a more sustainable future in industrial chemistry. As the momentum for sustainability increases, initiatives like these will undoubtedly inspire further research and revolutionize established practices in chemical synthesis.