In an exciting advancement for the field of chemistry, a group of researchers led by Professor Ingo Krossing at the University of Freiburg has unlocked new possibilities in the oxidation potential of positive ions. Traditionally constrained by the limitations of solvents and anions, the Krossing group’s innovative approach significantly enhances the potential for oxidation, showcasing potentials reaching up to +1.52 V versus the ferrocene/ferrocenium (Fc+/0) standard. This leap in electrochemical capacity can pave the way for groundbreaking applications in electrocatalysis and redox mediators, bringing forth both theoretical and practical implications in material science and inorganic chemistry.
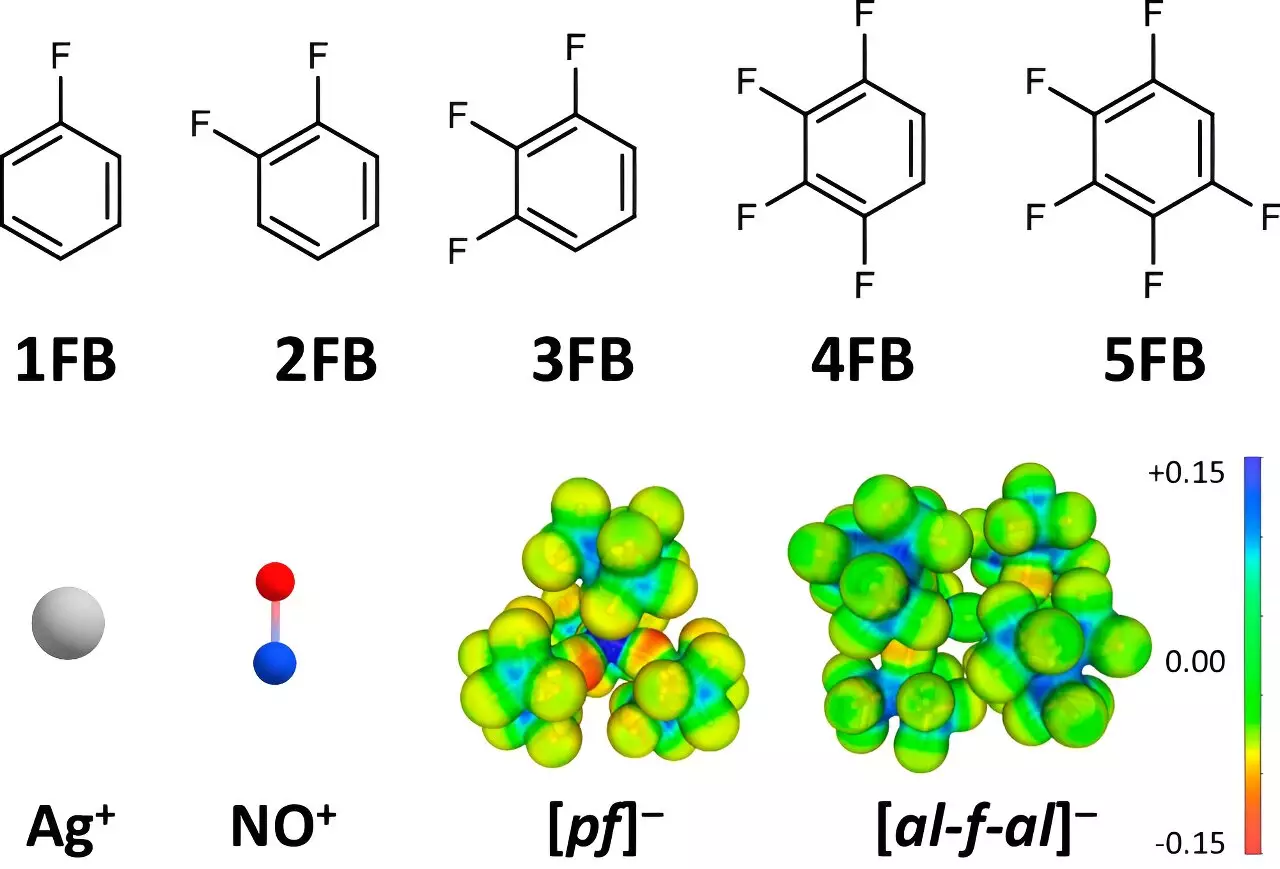
Oxidation potentials are fundamental to various chemical reactions, particularly in the context of electron transfer. The standard approach has largely revolved around utilizing well-known positive ions, such as silver (Ag+) and nitrosonium (NO+), as oxidizing agents. These ions exhibit strong interactions with their surroundings, primarily due to their small size and high charge density, which typically diminish their oxidation potential. The Krossing team’s research confronts this issue head-on by employing less interactive solvents and anions. Herein lies the essence of their methodology: by minimizing the engagement between the positive ions and their environment, they significantly enhance the ions’ oxidizing capability.
At the heart of this research is the concept of weakly coordinating anions (WCA) and solvents. By strategically selecting solvents such as fluorinated benzene derivatives, the research team expertly countered the strong interactions that usually inhibit oxidation. The utilization of these fluorinated compounds was not arbitrary; the associated dielectric constants were critically analyzed. Such insights facilitated the pairing of these solvents with positively charged ions, resulting in increased electron extraction capabilities.
Dr. Johannes Hunger from the Max Planck Institute for Polymer Research lent his expertise, revealing that fluorinated aromatic compounds demonstrate significantly higher dielectric constants compared to conventional solvents, like dichloromethane or acetone. This realization underlines the importance of solvent selection in optimizing oxidation potentials. Not only does the increased fluorination lead to reduced interactions between the solvent and the ions, but it also allows for the preservation of the ions’ reactivity, leading to previously unattainable oxidation reactions.
Complementing their electrochemical investigations, the team employed single-crystal X-ray diffraction to elucidate the solid-state structures of complexes formed by the solvents and positive ions. According to co-author Dr. Malte Sellin, these detailed structural analyses showcased how enhanced fluorination results in lower interactive forces, further establishing the foundations for engaging in unique chemical transformations. These findings emphasize the importance of understanding molecular architectures in both theoretical predictions and experimental implementations.
The implications of this research extend far beyond academic curiosity. With finely tuned oxidation capabilities, researchers can selectively target and manipulate chemical reactions, thereby facilitating a myriad of applications in various domains, including electrocatalysis and redox shuttles. The potential for creating new materials, as well as enhancing existing reaction pathways, presents numerous avenues for further exploration.
Ultimately, this research represents a remarkable fusion of old and new techniques—a classic problem in oxidation potentials approached through novel material chemistry. As scientists continue to delve into the intricacies of molecular interactions, the possibilities are rich and varied. The advancement led by Krossing’s team is not just a mere evolution in understanding oxidation; it is a testament to the power of innovative thinking in chemistry, heralding a future replete with new chemical phenomena waiting to be discovered.