In recent years, the evolution of energy storage technology has become a critical area of research, especially amid increasing concerns about safety and efficiency. Traditional lithium-ion batteries rely on liquid electrolytes that, while effective, present significant risks such as leakage, volatility, and thermal runaway. In pursuit of safer and more efficient systems, researchers have turned their attention to solid-state electrolytes, which offer a more stable and secure alternative. Nonetheless, the transition from conventional liquid electrolytes to solid-state counterparts is fraught with challenges, primarily related to conductivity and material stability.
Enhancing Conductivity through Structural Design
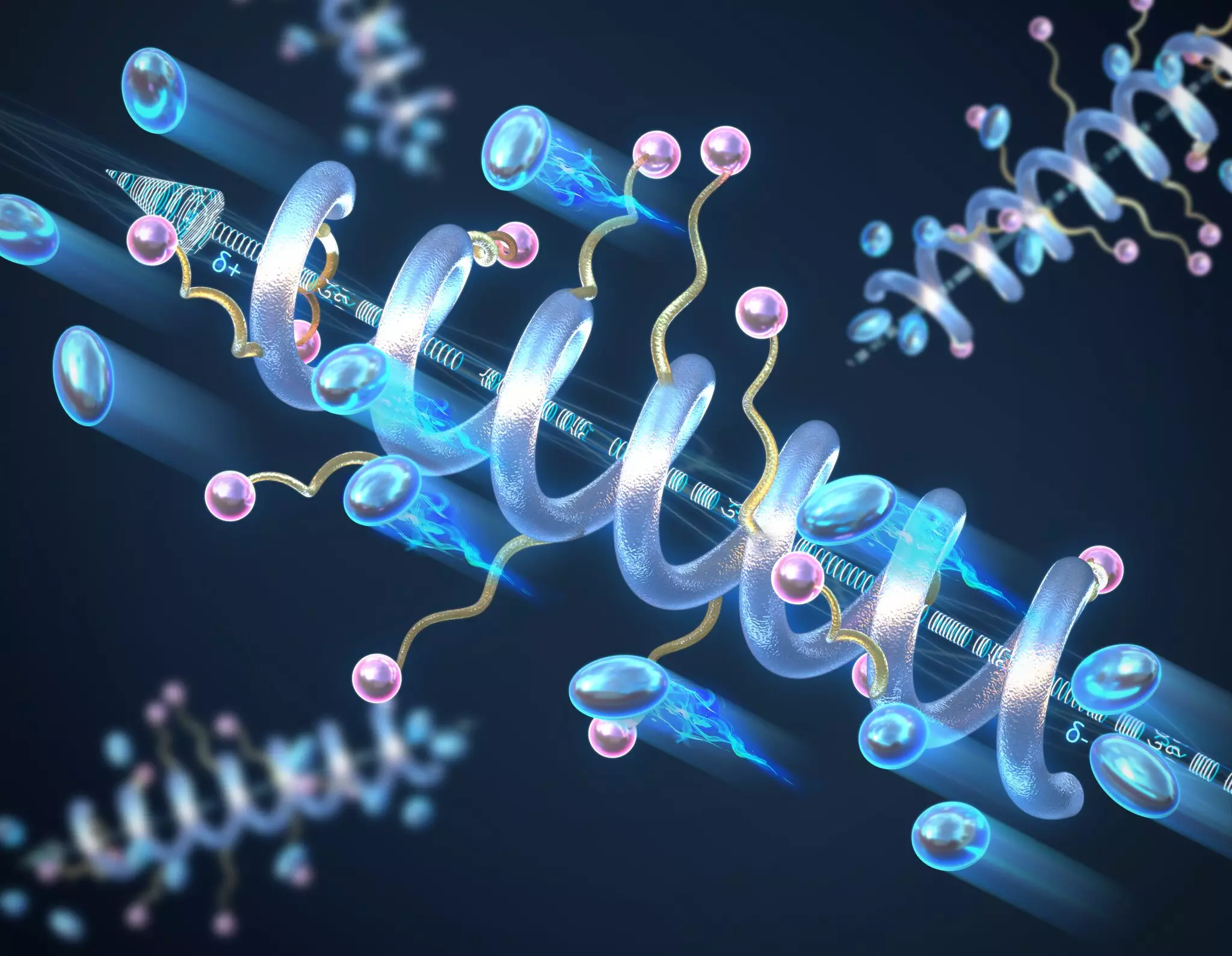
Innovations in materials science are essential to overcoming the limitations of current solid polymer electrolytes. Researchers at the University of Illinois Urbana-Champaign have conducted pivotal studies focusing on the structural characteristics that influence ionic conductivity in these materials. Their work centers on the helical secondary structure of peptide polymer electrolytes—an approach that diverges from the traditional “random coil” structures commonly observed in polymers.
By understanding and harnessing the helical arrangement, the team discovered that these structures significantly improve ionic conductivity. The significance of this finding cannot be overstated; the helical formation allows for efficient charge transport by creating a macrodipole moment. This moment results from the cumulative effect of individual dipole moments in the peptide units, enhancing the material’s dielectric constant and overall electrical performance.
One of the key revelations from this research is that the length of the helical structure plays a pivotal role in determining conductivity. Longer helices correlate with higher ionic conductivity, suggesting that the molecular architecture can be engineered to optimize performance. This opens new avenues for the design of solid-state electrolytes, as materials can be crafted with specific lengths and configurations to meet application needs.
Professor Chris Evans, who led this groundbreaking research, expressed enthusiasm for the project’s implications. By utilizing a naturally occurring structural motif—the helix found in biological peptides—for engineering non-biological materials, researchers are not only innovating but also broadening the scope of potential applications for these electrolytes.
Another significant advantage of the helical peptide polymers is their enhanced stability under diverse operational conditions. Unlike conventional random coil polymers, which may degrade under heat or voltage stress, the helical configuration remains intact. Evans noted that the robustness of the helical structure ensures that it does not break down prematurely, which is crucial for extending the lifespan of energy storage devices.
Furthermore, the ecological footprint of these materials adds another layer of appeal. The peptide-based materials are biodegradable and can be deconstructed safely through enzyme or acid treatment once they reach the end of their useful life. This not only mitigates environmental concerns associated with battery disposal but also allows for the recovery and reuse of monomer units after a separation process, promoting sustainability in energy storage technologies.
The research conducted at the University of Illinois Urbana-Champaign marks a significant advance in the quest for efficient and safe energy storage solutions. By focusing on the helical structure of peptide polymers, scientists have revealed a promising pathway towards the development of solid-state electrolytes that boast enhanced conductivity and stability compared to traditional materials. As the world increasingly pivots towards renewable energy sources, the integration of such innovative materials into next-generation solid-state batteries could transform energy storage practices, enabling safer and more sustainable energy systems for the future. The findings of this study resonate not only within the scientific community but also highlight the broader societal implications of advancing battery technology.