Polymers are ubiquitous materials that play vital roles across diverse industries, from healthcare to technology. When considering their composition, one can visualize polymers as intricate trains, where monomers serve as individual cars connected through chemical bonds, much like couplings that keep trains intact. Recent advancements in polymer chemistry have opened doors to creating more versatile and functional materials. A recent study from Scripps Research, published in *Nature Synthesis*, has proposed an innovative reaction that employs nickel as a catalyst, paving the way for the synthesis of exceptional monomer units. This article will delve into the significance of these findings, the methodology employed, and the potential impacts on future applications of polymers.
Traditional polymerization techniques have largely depended on a limited set of monomers with similar chemical characteristics. Such constraints can stifle innovations in material properties and functionalities. The Scripps Research team, led by Dr. Keary Engle, has developed a novel reaction mechanism that enhances the structural diversity of monomers used in polymer synthesis. This new method allows for the introduction of distinct functional groups to monomers, tailoring the resultant polymers to meet specific needs such as increased elasticity or solubility.
The underpinning concept of the study lies in utilizing nickel, a more abundant and less expensive metal catalyst compared to other conventional catalysts. By modifying a base molecule with nickel, the researchers successfully synthesized new monomers that hold considerable promise for a range of applications, including drug delivery systems and energy storage solutions. The innovation lies not only in the creation of these monomers but also in the potential for scalable production, allowing for practical implementation in various sectors.
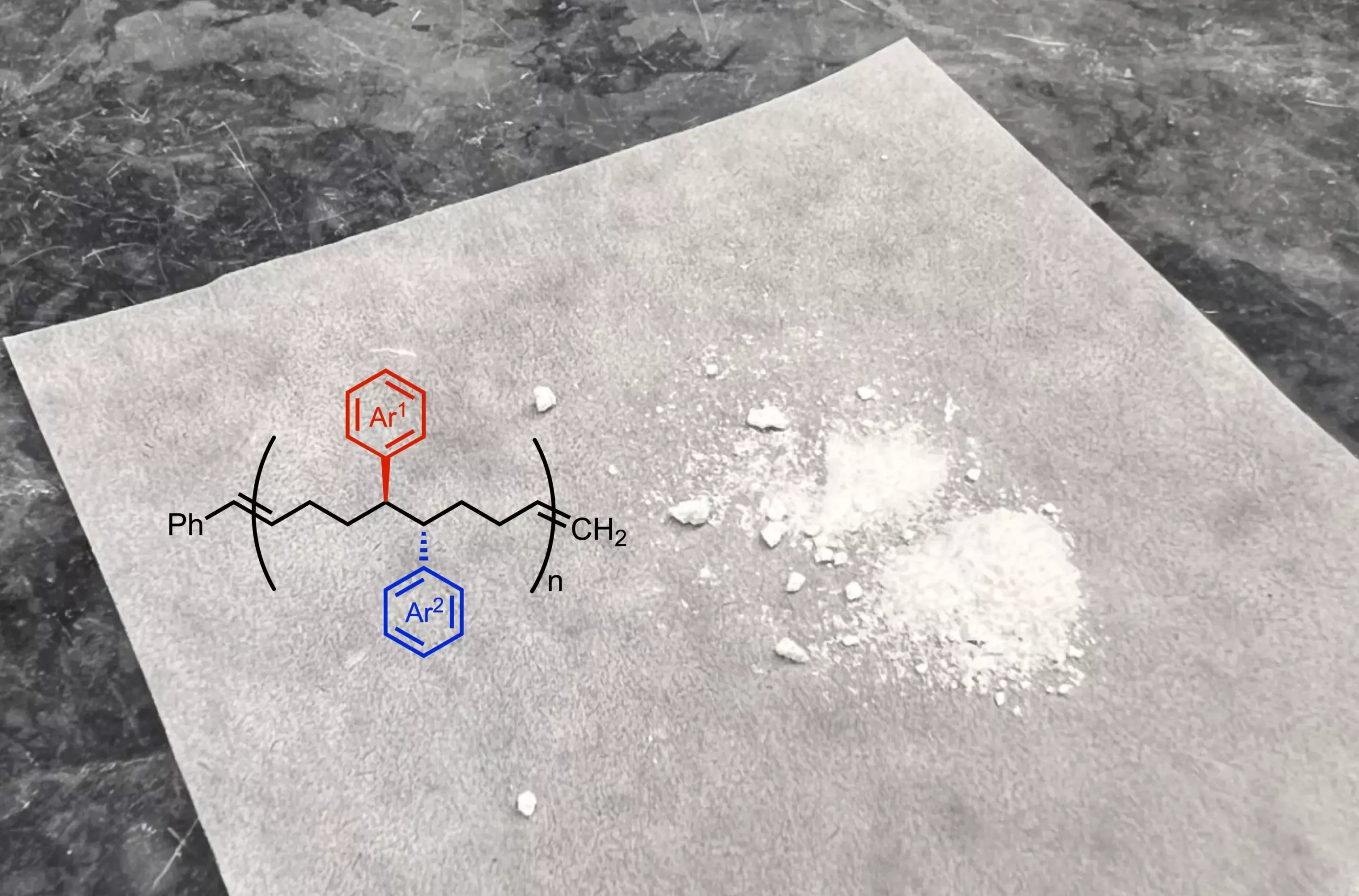
At the heart of this research lies a well-designed chemical reaction that transforms a basic structure into a complex monomer. The engagement of nickel as a catalyst in this reaction is a pivotal advancement, as it introduces two new functional groups that significantly alter the properties of the polymer. Anne Ravn, a key researcher in the project, emphasizes that the structural modifications allow for a closer placement of functional groups, enabling unique interactions within the polymer chains that can lead to significantly different material characteristics.
This reconfiguration of monomers affirms that the chemical compositionality of polymers can be “decorated” beyond conventional means, expanding the toolbox for chemists to craft polymers with desired properties. The innovative aspect of this research hinges on its ability to manipulate polymer backbones fundamentally, broadening the horizon for engineering materials with selective functionalities that conventional methods struggle to achieve.
Interdisciplinary Collaboration for Enhanced Outcomes
The study’s execution was not a solitary endeavor; it was a collaborative effort involving experts from Scripps Research, Georgia Institute of Technology, and the University of Pittsburgh. This interdisciplinary approach has proven essential, allowing insights from various fields of research to converge. The joint effort not only enhances the study’s robustness but also establishes a foundation for future explorations into polymer science.
Through the collaboration, the team successfully implemented another chemical reaction to polymerize the newly developed monomers into complex structures. This synthesis method holds the potential for rapid production of customizable polymers that can meet specific criteria dictated by end-user needs. The impact of interdisciplinary science is clear: varied expertise leads to innovative methodologies and solutions in the complex world of material science.
Environmental Considerations and Future Directions
The ecological implications of polymer production have become an area of increasing concern as industries strive for sustainability. The research team acknowledges this challenge and highlights the environmental benefits of using nickel over rarer metals. Nickel’s abundance not only reduces costs but also makes the polymer production process more sustainable.
Moreover, Ravn’s team is actively investigating methods to enhance the sustainability of the produced polymers, particularly focusing on ways to degrade long-chain polymers back to their initial building blocks. This cyclical reuse framework promises to mitigate plastic waste issues that pervade numerous environments, aligning with global sustainability goals.
The research conducted by Scripps Research signifies a monumental leap in polymer chemistry, showcasing how innovative use of nickel as a catalyst can redefine material synthesis. By creating a diverse array of functional monomers and promoting interdisciplinary collaboration, the study lays a foundation not only for advanced materials but also for eco-conscious production methodologies. As scientists continue to explore the capabilities of these new monomers and their potential applications, the future of polymer science appears promising, with prospects of enhancing existing technologies and pioneering new solutions in critical areas such as healthcare and energy.