Microplastics have emerged as a significant environmental concern, infiltrating waterways, oceans, and ecosystems globally. With their ubiquity comes a range of questions regarding their behavior and fate in different environmental conditions. A recent study published in *Environmental Science & Technology* provides illuminating insights into how the freezing and thawing processes impact microplastic behavior. This article will dissect the findings of this research, discuss its implications for environmental science, and highlight the nuances of microplastic interactions in aquatic environments.
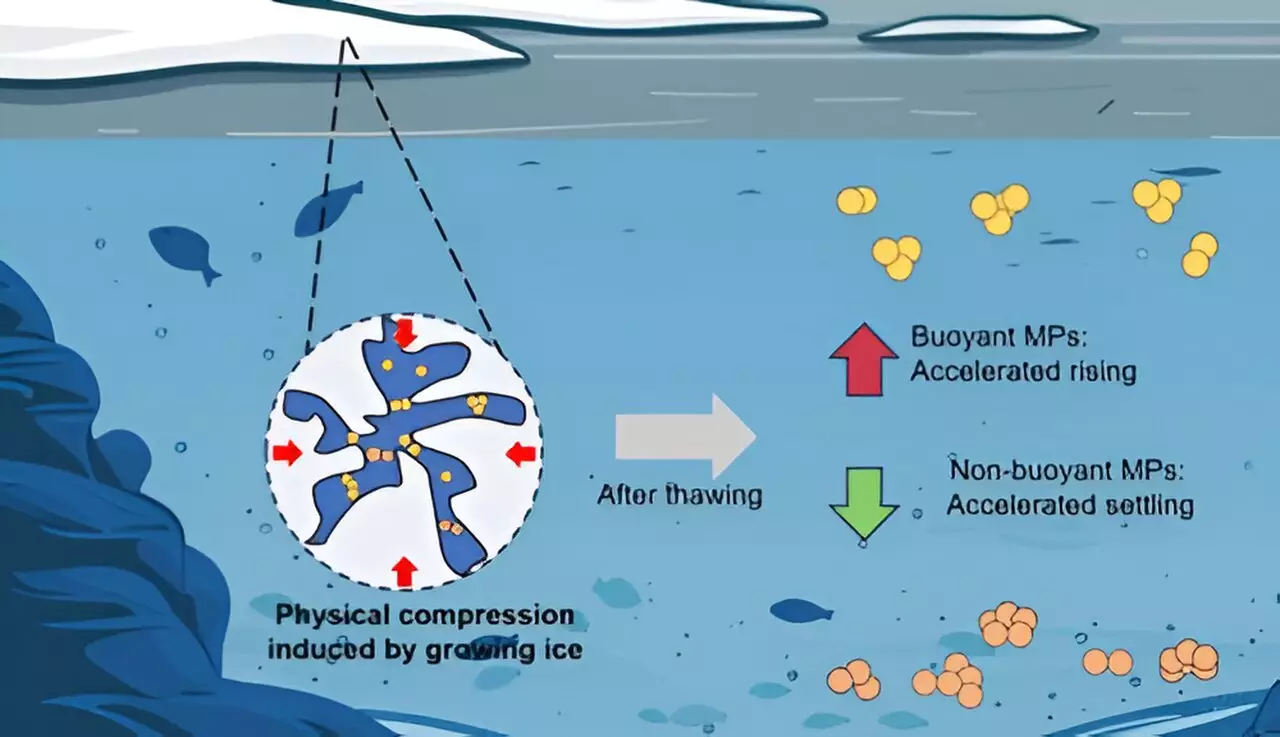
As ice forms on bodies of water, it captures tiny plastic particles, effectively trapping them at the surface. However, the melting process does not merely release these microplastics unchanged; instead, research indicates that the particles may undergo significant transformations in size and buoyancy. This change suggests that the freezing process may play a pivotal role in determining the environmental consequences of microplastics. The study, led by Chunjiang An and his colleagues, found that the dynamics of microplastics can vary remarkably depending on the polymer type. Polyethylene (PE), for example, is buoyant and floats, while other polymers like polyurethane (PU) and polytetrafluoroethylene (PTFE), which are denser than water, gravitate towards the sediment.
This distinction in behavior emphasizes that not all microplastics are created equal. Their journey and impact within aquatic ecosystems are intricately tied to their chemical composition. Understanding these variances is crucial, as it allows for a more nuanced approach in addressing the pollution crisis posed by microplastics.
To explore how freezing impacts microplastic particles, An’s research team engaged in meticulous laboratory experiments. They began with different types of plastic particles, sized between 6 and 10 micrometers, suspended in freshwater and saltwater solutions. These samples were subjected to a freezing process lasting 24 hours, followed by thawing. The results were compelling; they observed notable increases in the size of the particles after being frozen. Specifically, PE particles enlarged considerably—by 46%—while PU particles saw a more modest uptick of 9%.
These findings indicate that different polymers react distinctively under similar conditions. PE’s significant expansion can be attributed to its hydrophobic nature, which repels water and allows for more substantial accumulation, leading to greater size increase after freezing. In contrast, PU’s hydrophilic properties promote dispersion, preventing the same level of enlargement. This phenomenon not only illustrates the divergent physical properties among various plastics but also hints at the broader implications these changes have on the sedimentation patterns in water bodies.
Interestingly, as salinity levels increased in the experimental samples, the freezing process did not yield any noticeable changes in particle size. Researchers posited that the creation of brine channels within the freezing ice facilitated the microplastics’ movement, preventing them from merging into larger clumps. This observation underscores the importance of environmental conditions—such as salinity—in shaping the fate of microplastics.
Given that marine environments often have varying salinity levels, these findings could indicate that the behavior of microplastics in estuarine or coastal areas may differ significantly from that in freshwater settings. Identifying these environmental variables helps create a more comprehensive understanding of microplastic dynamics and potential strategies for mitigation.
The insights gleaned from this study present a need for proactive environmental policies. As the research suggests, rapid freezing may expedite the settling of microplastics into sediments, potential contributing to their long-term accumulation in freshwater systems. This issue raises alarms about environmental degradation and emphasizes the necessity for water management strategies that consider the implications of climate change, particularly warmer winters and changing ice dynamics.
While this study is foundational, researchers acknowledge the limitations posed by its relatively short freezing period. More prolonged observational studies mirroring natural conditions are necessary to enhance our understanding of microplastic behavior over time. As the global community continues to grapple with the consequences of microplastic pollution, informed research and policy decisions will be paramount in safeguarding aquatic ecosystems.
The ongoing investigation into the behavior of microplastics in frozen environments offers invaluable insights into their environmental impact and potential avenues for effective management practices. As the iceberg of knowledge on microplastics slowly surfaces, it is imperative to remain vigilant and proactive in addressing this pressing global concern.