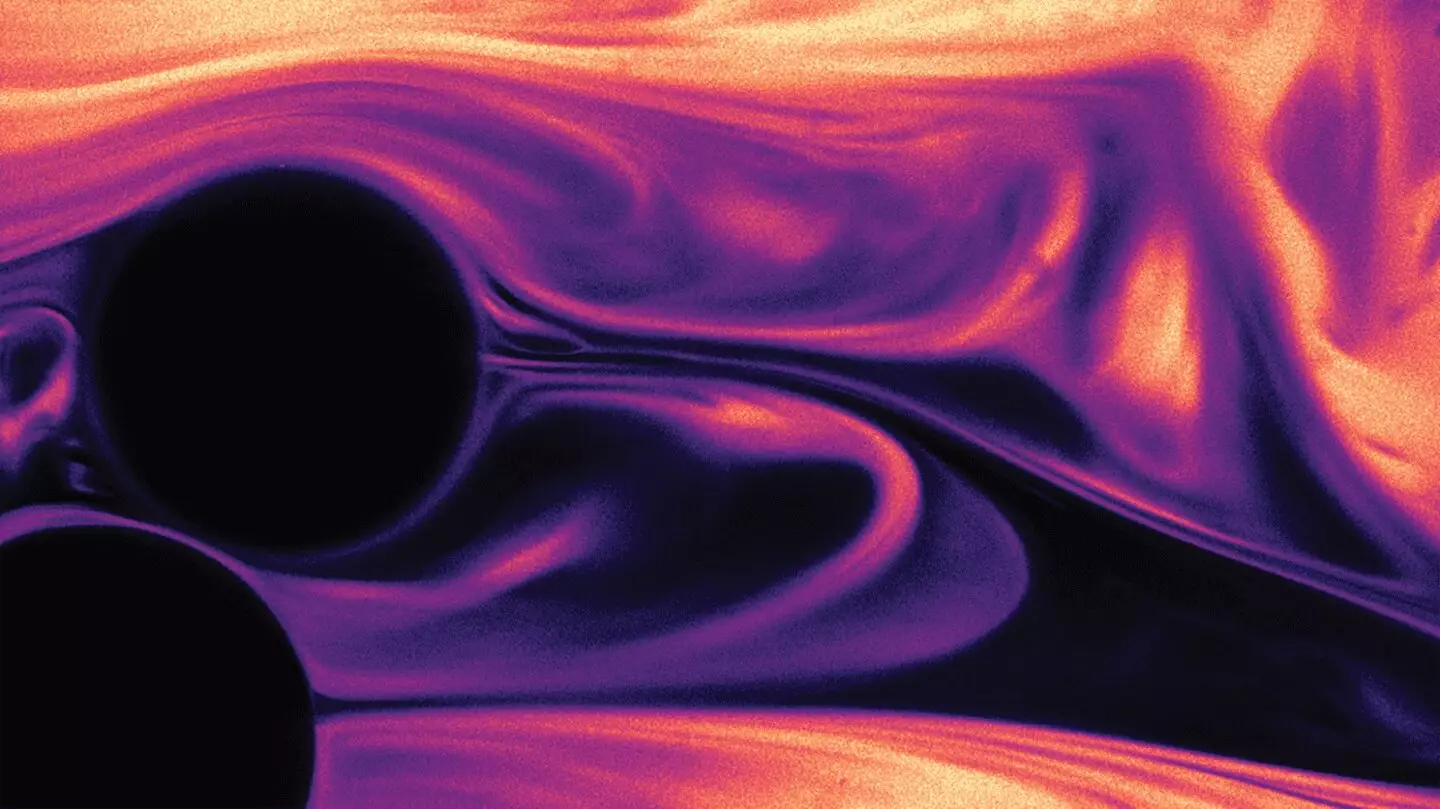
Imagine witnessing the swirl of cream as it cascades into coffee, forming intricate patterns similar to the tumultuous bands of Jupiter. This mesmerizing scene serves as a reminder of how fluid dynamics can captivate our senses. Yet, the mesmerizing swirl conceals an intricate science that offers more than just aesthetic beauty. Mixing is a fundamental process critical to various industrial applications, ranging from food production to advanced chemical manufacturing. But achieving the right kind of mixing is not as simple as it may seem.
In a typical coffee mug or cocktail shaker, turbulent flows foster a chaotic environment that facilitates the rapid amalgamation of liquids. Conversely, scenarios like those encountered in industrial settings, particularly within packed beds—as seen in processes such as espresso brewing—present unique challenges. In these environments, fluids struggle to blend due to limited space and poor turbulence, rendering effective mixing a complex yet essential task.
The Challenge of Packed Beds
Packed beds are crucial in numerous fields, including plastics production and environmental remediation. In such contexts, the fluid must navigate through a bed of compacted particles, maximizing surface contact but limiting turbulence. The result is a slow mixing process that ultimately hampers chemical reaction rates. Given that reaction efficiency can dictate the overall output of industrial applications, researchers have long sought innovative methods to enhance fluid mixing.
Historically, modifications of grain geometry have been the go-to solution for improving mixing efficiency, requiring costly reengineering that many industries may find impractical or expensive. This struggle illustrates a paradox in engineering: the pursuit of optimal designs often overshadows the exploration of simpler, more elegant solutions.
The Princeton Solution: Polymers Over Geometry
Enter the groundbreaking research conducted by Princeton University’s Department of Chemical and Biological Engineering. Led by Christopher Browne under the guidance of Sujit Datta, the research team explored a novel approach to tackle the mixing dilemma by introducing springy polymers into the fluid. Unlike traditional methods that focused on altering geometry, the inclusion of these flexible polymers effectively mimicked turbulent mixing within the constraints of packed particles.
The polymers function as microscopic agents of chaos, stretching and recoiling with each movement through the pores in the packed bed. As they interact with the fluid, they generate a localized mixing environment akin to that found in larger turbulent volumes, fundamentally changing the game for chemical reaction rates. This surprisingly simple technique has the potential to enhance reaction speeds by an astounding factor of ten, offering a promising avenue for improving efficiency across a wide range of industrial and environmental applications.
Engineering Opportunities and More
Browne’s journey into this breakthrough started during his early graduate studies and evolved through a series of complex challenges, demonstrating the importance of persistence in scientific inquiry. Each complication unraveled new layers of understanding that ultimately contributed to the success of their findings. The dynamic power of fresh perspectives cannot be overstated; often, stepping back from a problem allows for innovative insights that may have otherwise been eclipsed by the demands of active research.
Datta emphasizes that while fluid mechanics will always hold an allure for academics and engineers alike, this research has real-world relevance. Utilizing common polymers, similar to materials found in everyday products, paves the way for practical applications that can be readily implemented. This turns the challenge of mixing into a manageable phenomenon, promising scalability that previous geometrical methods could not provide.
Implications for Industry and Environmental Science
The ripple effects of these findings extend beyond mere industrial efficiency; they could potentially revolutionize environmental practices as well. With improved mixing capabilities, processes related to carbon capture might become significantly more effective, addressing pressing environmental concerns through better management of pollutants. Moreover, industries reliant on chemical reactions would find new opportunities for reducing costs while enhancing output—a dual benefit that could reshape economic landscapes.
In an era where efficiency and sustainability have become paramount, this research stands as a testament to the ingenuity of modern chemical engineering. The marriage of science and practicality not only showcases the beauty of fluid dynamics but also highlights a pathway toward innovative solutions that address not just industry needs, but also environmental issues that impact us all.
The discovery at Princeton is a reminder that solutions do not always demand high costs or complexity. Sometimes, true innovation arises from understanding fundamental principles in new ways—an exciting prospect that could very well dictate the future of engineering and environmental science.