Ice is a remarkable substance, often seen as a static solid formed from water. However, the reality is much more complex. Ice seldom exists in isolation; it is usually enveloped by a layer of liquid water. This interplay between solid ice and its liquid counterpart is fundamental to various natural processes—from the formation of snowflakes to the delightful sensation of licking ice cream on a hot day. To truly grasp the phenomena surrounding ice, we must unravel the intricate interactions at the ice-liquid interface. A groundbreaking study conducted by researchers at Kobe University and the Institute for Molecular Science has taken significant strides in illuminating this mysterious juncture.
Innovative Approaches to Observing Ice
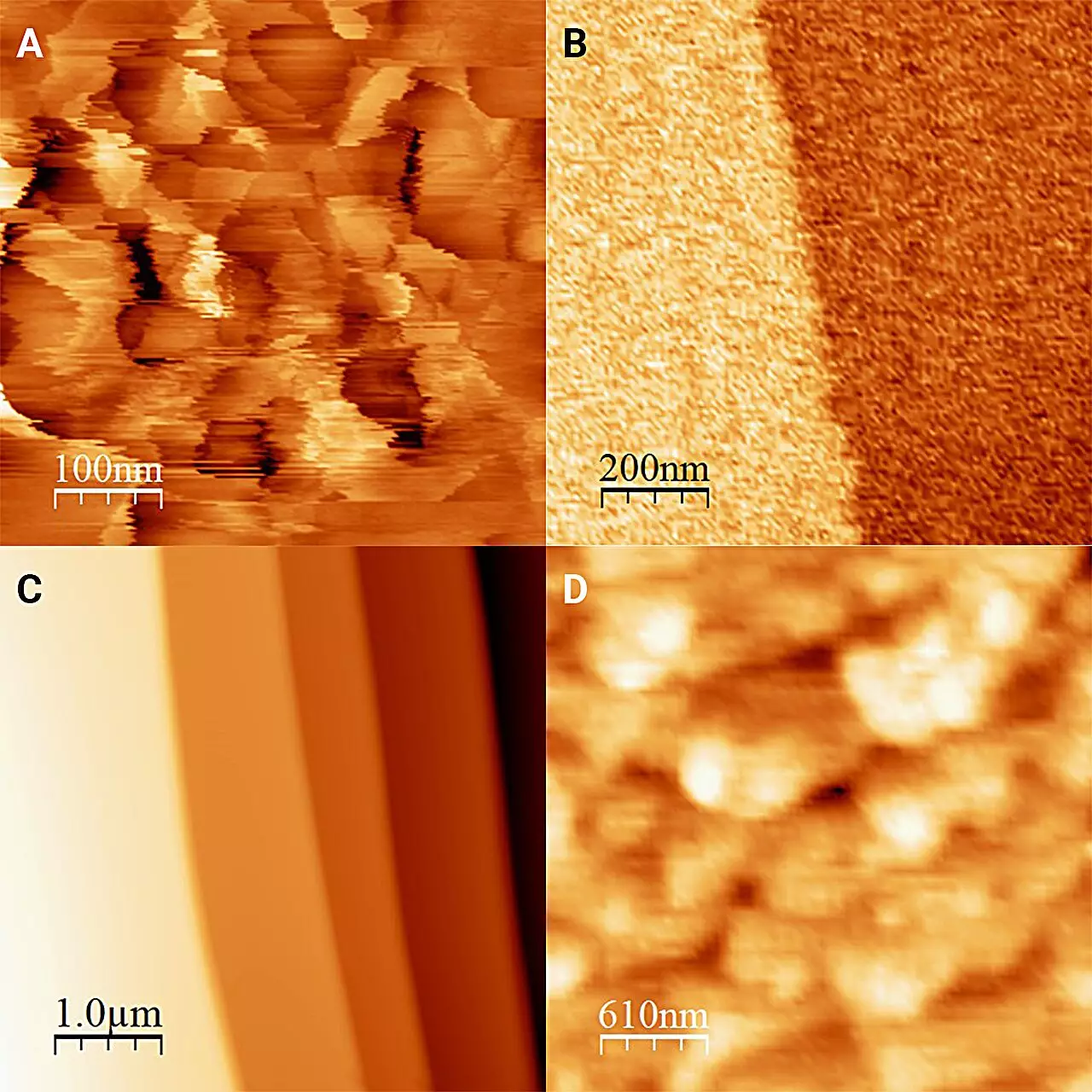
The research team’s ambition was not merely to investigate the ice alone but to observe how it behaves in conjunction with liquid. Traditional methodologies have faced limitations due to the inherent volatility of ice transforming rapidly into water and vice versa. This presents a substantial hurdle in observing their direct interaction. However, the researchers employed an ingenious strategy by immersing ice in antifreeze cooled below 0°C. This novel approach prevented the ice from melting, allowing the researchers to maintain a consistent interface for observation. As Onishi Hiroshi, the study’s lead researcher, explains, this allowed for clearer insights into the dynamics at play.
Achieving stable observations, however, was no easy feat. The researchers faced numerous setbacks, requiring a cooling box to regulate the entire microscopic system’s temperature and ensure the precision measuring instruments would function properly under sub-zero conditions. Their resolve and ingenuity allowed them to push the boundaries of what was previously possible in ice research.
Unveiling Unexpected Findings
The results, published in The Journal of Chemical Physics, revealed fascinating differences in the structure of the ice at its interface with liquid. Without the surrounding liquid layer, ice forms “frost pillars,” small protrusions approximately 20 nanometers tall. Yet, when encased in antifreeze—specifically 1-octanol—the surface of the ice appeared remarkably flat, with only occasional molecular-level steps. This finding suggests that the interaction with the antifreeze caused a process of partial dissolution and recrystallization, radically transforming the surface characteristics of the ice.
Moreover, the study ventured into exploring various types of liquids that could serve as antifreeze. While all the examined liquids were alcohols sharing similar physical properties, the resulting surfaces of ice reflected not just quantitative but qualitative differences, emphasizing the necessity of direct measurement methodologies. These variations underline the complexity and specificity of the ice-liquid interface dynamics, shedding light on how minor changes in the surrounding environment can drastically impact the interactions at the molecular level.
Rethinking Ice’s Qualities
An additional revelation from the study was the hardness of the ice surface in antifreeze, which turned out to be significantly harder than previous estimations derived from traditional methods. This conclusion challenges prior assumptions about the nature of ice, urging scientists to reconsider the characteristics of ice when affected by different environments. As researchers continue to probe the ice-liquid interface, each discovery not only enhances our understanding but may also stimulate further inquiries into other material properties and their applications in various scientific fields.
A Vision for Future Research
While the Kobe University team’s findings lay a robust groundwork, they recognize the work is far from complete. Their future aspirations focus on attaining even greater microscopic resolution, potentially allowing observations down to the level of single water molecules. This ambitious goal points to a continually evolving understanding of the intricate dynamics at play between ice and liquid. By applying advanced measurement techniques beyond atomic force microscopy, the researchers hope to deepen our knowledge of these fundamental interactions.
The study of ice is far more than an academic endeavor; it cuts across numerous real-world applications and natural phenomena. From climate science, where ice dynamics are crucial in understanding global warming, to industrial applications involving freezing processes or cryogenics, the implications of these ice-liquid interaction insights are vast and profound. As science continues to explore the intricate dance between these two states of water, we may uncover mysteries that alter our understanding of both ice and liquid water—and their observations in nature at large.