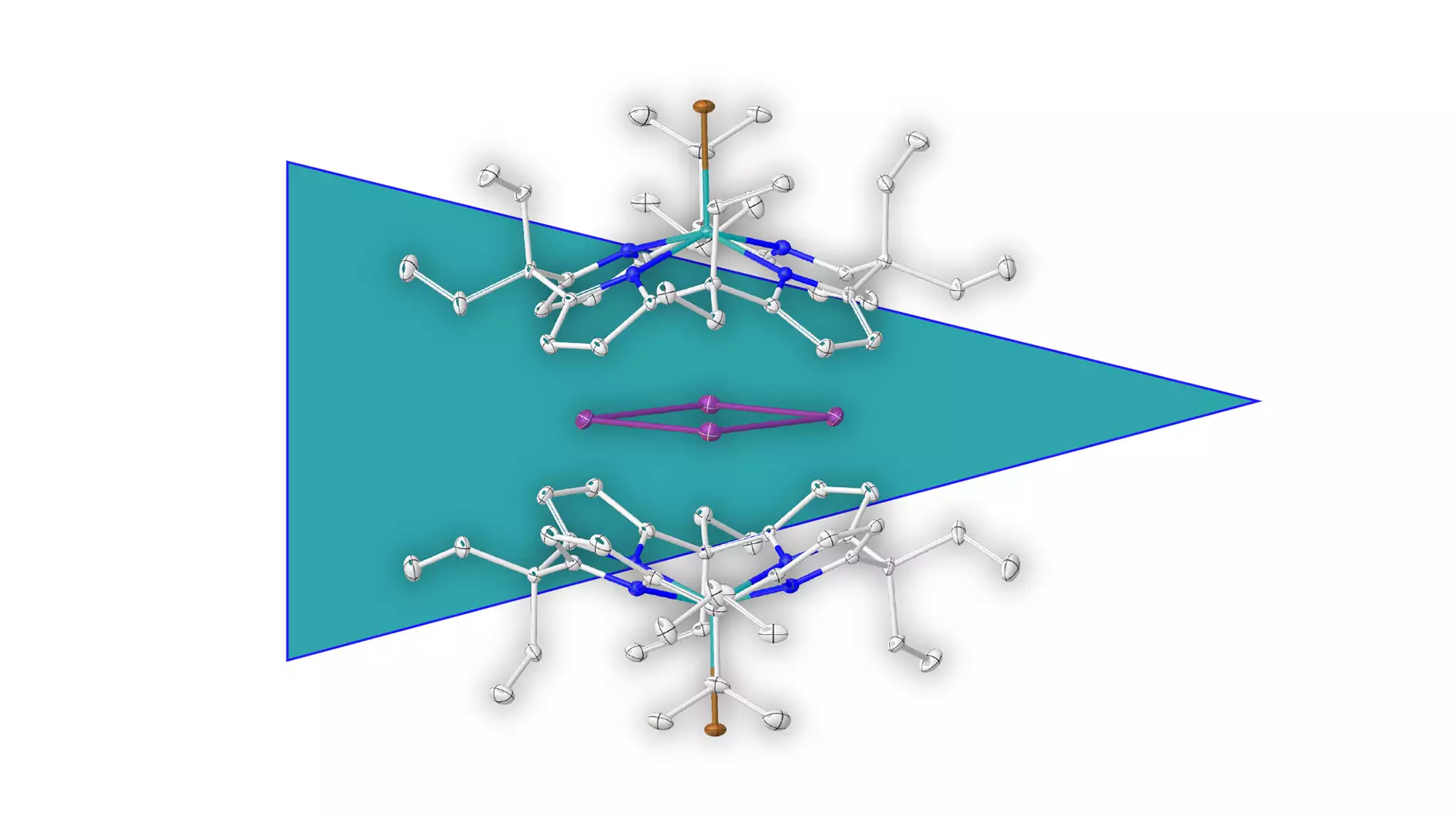
Aromaticity is a cornerstone of organic chemistry, defining a unique class of compounds that feature cyclic structures exhibiting resonance stability. Traditionally associated with carbon atoms, the concept has primarily revolved around organic molecules that exude distinct aromatic properties—often tied to their pleasant fragrances. However, the paradigm is shifting. A remarkable breakthrough by researchers at Heidelberg University has unveiled a new frontier in aromatic compounds: pure metal aromaticity, particularly involving elemental bismuth. This discovery is not just a mere extension of existing knowledge; it heralds the dawn of a new understanding of chemical structures that could fundamentally alter how we perceive aromaticity.
Pioneering Research in Metal Aromatics
Led by Prof. Dr. Lutz Greb, the research team has made strides in isolating and characterizing a metal ring composed exclusively of bismuth atoms, a feat previously deemed unattainable. This innovative achievement emerged through the use of a novel supramolecular stabilization approach—encapsulating the positively charged metal ring in a negatively charged molecular shell. This ingenious method prevents breakdown reactions that could otherwise undermine the stability of the metal structure. The implications of this discovery extend far beyond mere academic interest; they invite reevaluation of existing theories surrounding stability and aromaticity within the realm of metal-containing compounds.
Implications for Fundamental Chemistry
This groundbreaking work does more than add an intriguing chapter to the book of aromatic compounds; it challenges and potentially reshapes foundational principles in chemistry. The researchers propose that such aromatic rings comprised entirely of metals stand to enhance our understanding of charge transport phenomena in metals, opening up avenues for future research. The ability to manipulate and study aromaticity in a new light could significantly impact diverse fields, from materials science to computational chemistry.
Furthermore, as Greb himself suggested, this methodology of stabilization may serve as a general tool in various contexts beyond aromatic compounds. This principle could lead to the exploration of other positively charged rings and cages, offering new vistas for synthetic chemistry and materials development.
Challenges and Future Directions
Despite the excitement surrounding this discovery, it is essential to approach this breakthrough with a critical lens. Unlocking the secrets of metal aromaticity may pose its own set of challenges, particularly in replicating the conditions necessary for stabilization and isolation. Scientists will need to navigate a host of reactions and interactions that could complicate further research. Nevertheless, the potential benefits of success outweigh these challenges, suggesting a robust future for this line of inquiry.
The isolation of pure metal aromaticity stands to create ripples in the field of chemistry, inviting both skepticism and hope for transformative advancements. As researchers continue to unravel the complexities of these newly discovered properties, it’s evident that the journey into the future of aromatic compounds is just beginning. This groundbreaking work signifies not only an expansion of our understanding but also the promising potential to revolutionize both theoretical and practical dimensions of materials science.