The realm of regenerative medicine is on the brink of transformative advances, specifically with a pioneering research endeavor at the University of Virginia. Researchers Liheng Cai and his Ph.D. student Jinchang Zhu have made significant strides in creating the first building blocks for human-compatible organs with remarkable precision and versatility. Having successfully engineered biomaterials that closely mimic the mechanical properties of various human tissues, their breakthrough could redefine the landscape of 3D bioprinting. Published in July 2023 in the renowned journal *Nature Communications*, this research has profound implications for the future of organ transplantation and disease therapy.
Understanding the Breakthrough: DASP Technology
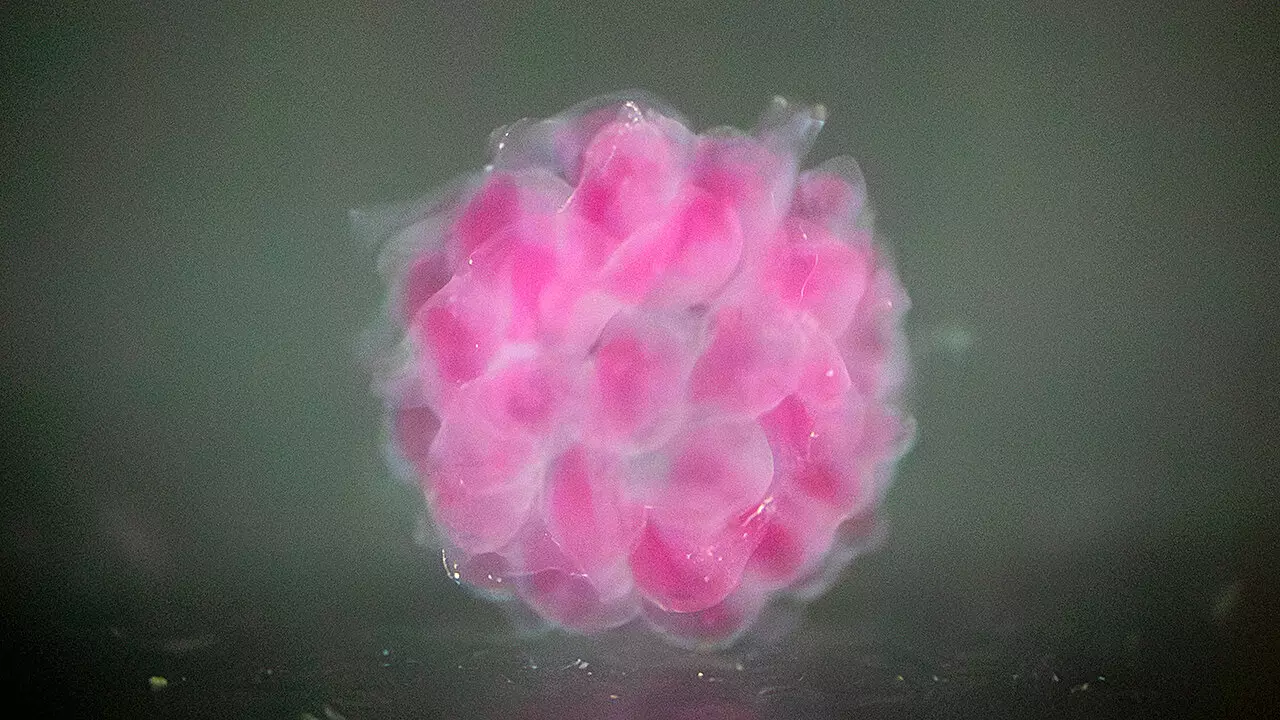
At the core of this innovation is the digital assembly of spherical particles (DASP), a novel bioprinting technique developed by Cai and Zhu. Unlike conventional methods, DASP employs a unique strategy where particles of biomaterial are deposited into a supportive water-based matrix. This cutting-edge approach functions similarly to voxels—akin to 3D pixels used in digital imaging—allowing for the construction of highly sophisticated 3D structures. According to Zhu, this technology represents a significant leap from existing bioprinting techniques, opening doors to create complex organoid systems that can replicate human tissue functions.
This breakthrough is not merely an academic curiosity. The implications reach far into the clinical domain. Organoids created through this process can serve as advanced models for studying disease progression, ultimately aiding in the quest for effective treatments. By harnessing the power of DASP, researchers can create tailored models that simulate real human tissues, thus paving the way for enhanced therapeutic strategies.
Key Features of the Biomaterials
What sets Cai and Zhu’s hydrogels apart are their unique properties. These polymer-based hydrogels are designed to emulate human tissue by manipulating the molecular structure of single-molecule monomers. This intricate engineering allows for a double-network hydrogel that is not only strong but also highly adjustable to mimic specific physical characteristics of human tissues. The low toxicity and high biocompatibility of these hydrogels signify a substantial advancement over traditional bio-inks, ensuring that the encapsulated human cells thrive in this engineered environment.
Crucially, their approach incorporates encapsulated human cells within these hydrogels, thus creating a more authentic representation of living tissues. This innovation stands to benefit multiple streams of research, from fundamental biological studies to practical applications in disease modeling and drug screening.
Advancements with DASP 2.0
Building on their foundational work described previously in *Advanced Functional Materials*, Cai and Zhu have now introduced DASP 2.0. This latest iteration implements “click chemistry,” which allows for rapid cross-linking of molecular structures, propelling the process of turning liquid droplets into an elastic gel. This transition occurs in merely 60 seconds, a feat made possible by the development of a sophisticated multichannel nozzle. This nozzle is crucial as it mixes hydrogel components in real-time, aligning with the fast-paced dynamics of the cross-linking process.
The ability to deposit large droplets from a fast-moving nozzle ensures that the resultant hydrogels can replicate the mechanical characteristics of human tissues, such as elasticity and stiffness. As Cai articulated, the precise manipulation of these viscoelastic voxels is both a fundamental and technological challenge in modern bioprinting, and DASP represents a pivotal solution.
Future Implications: From Theory to Practice
It’s evident that the ramifications of this innovation extend beyond academic boundaries. If fully realized, the applications of DASP technology can revolutionize various aspects of healthcare, including artificial organ transplants and advanced drug screening methodologies.
Imagine a future where patients in need of organ transplants no longer face the agonizing wait for a compatible donor. With the potential to print functional organs on demand, medical professionals could transform lives while significantly mitigating risks associated with transplant procedures. As researchers continue to refine this technology, it presents a promising frontier in healthcare, enabling personalized medicine and precise therapeutic interventions that were once mere concepts on the horizon.
This research underscores the importance of innovation in the field of bioprinting, with a clear trajectory leading toward improved patient outcomes and groundbreaking medical solutions. The unwavering dedication to translating scientific advancements into real-world applications signifies a compelling leap toward a future where organ shortages may become a relic of the past.