Carboxylic acids are fundamental to the framework of organic chemistry and find their applications in a spectrum of pharmaceuticals, including widely-used drugs like aspirin and ibuprofen. The integration of fluorine atoms into these acids has the potential to significantly enhance their properties, leading to more effective therapies. However, the endeavor to add fluorine usually involves complex and multi-faceted synthetic routes that can be tedious and time-consuming, thus presenting a barrier to innovation in drug design and development.
Recent Breakthrough in Synthesis
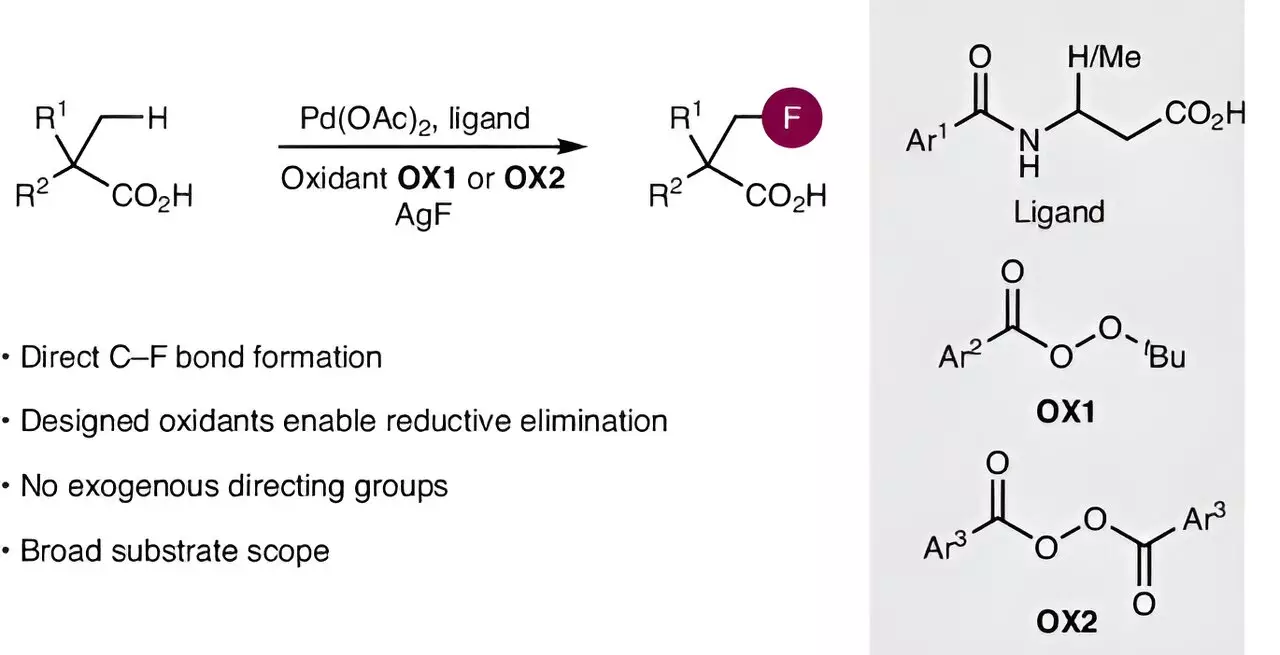
An international collective of chemists, including a research team from the Otto Diels Institute of Organic Chemistry at Kiel University, has recently published a pioneering method aimed at streamlining fluorine integration into aliphatic carboxylic acids. Their work, highlighted in the esteemed journal Nature Synthesis, presents a novel approach that circumvents the previously complicated synthesis process. By utilizing advanced catalysts and a cleverly designed oxidizing agent, they have made significant strides in overcoming two fundamental challenges in chemistry: activating usually inert carbon-hydrogen bonds and forming robust carbon-fluorine bonds.
Engineered Catalysts and Innovative Reagents
At the core of this breakthrough lies the cleverly engineered palladium catalysts, responsible for the crucial activation of carbon-hydrogen bonds, a feat that has baffled chemists for years. With assistance from earlier studies on catalyst optimization, this team was able to create particularly efficient catalysts tailored for their specific reaction demands. However, the formation of carbon-fluorine bonds posed an additional hurdle. Traditional methods proved inadequate, prompting the team led by Professor Manuel van Gemmeren to devise a creative solution involving a new oxidizing agent. This reagent plays a pivotal role in promoting the selective transition to form the desired carbon-fluorine bond, demonstrating that synergy in catalyst and oxidant design can yield previously unattainable reactions.
A New Paradigm in Chemical Synthesis
The implications of this innovative synthesis methodology extend beyond the immediate benefits of efficiency and simplicity in the laboratory. By demonstrating a unique reaction pathway, this research paves the way for other synthetic processes that previously hampered chemists. The potential for wider application suggests that this could be a transformative moment in the world of synthetic organic chemistry. Future studies may find that the principles honed in this research can be adapted and applied to various fields beyond pharmaceuticals, opening further avenues for chemical innovation and applicability.
Impact on Pharmaceutical Development
With carboxylic acids and fluorinated compounds both being cornerstones in pharmaceutical development, this new method heralds a plethora of opportunities in drug design. The ability to directly introduce fluorine atoms into complex molecular frameworks without the traditional drawbacks of synthetic labor is not just an improvement—it’s a paradigm shift. It empowers researchers to generate novel compounds with the potential for improved therapeutic efficacy and reduced side effects. With this advancement, the landscape of pharmaceutical research is set to evolve rapidly, introducing a quantum leap in delivering new, effective treatments to tackle unmet medical needs.