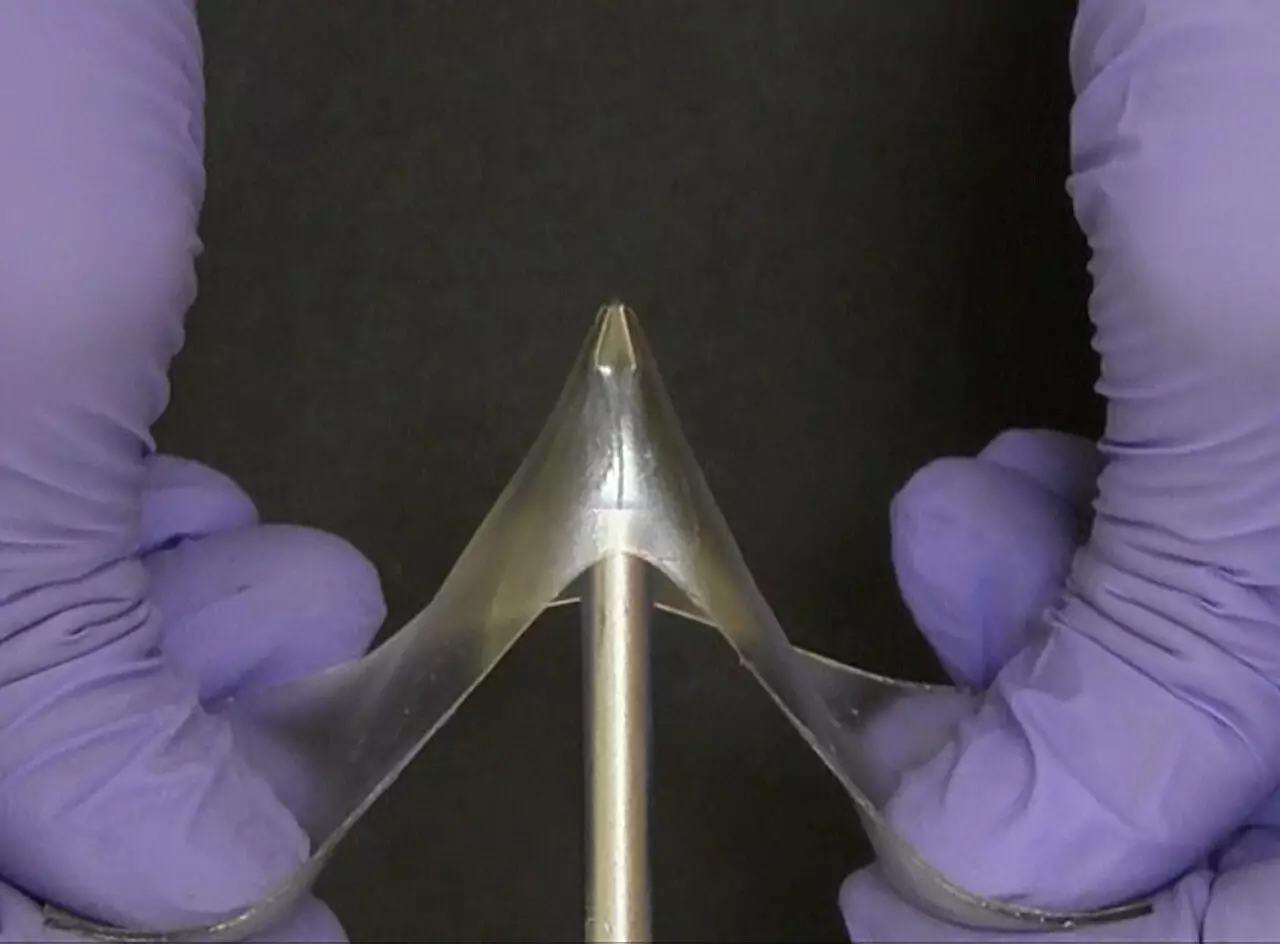
In the realm of material science, a remarkable innovation has emerged that challenges long-standing distinctions: glassy gels. These materials are redefining what we consider possible by combining the brittle firmness of glassy polymers with the fluid flexibility of gels. Traditionally, glassy polymers such as those used in windows or water bottles have been characterized by their rigidity and propensity to crack under stress, while gels—think contact lenses or biofilms—are celebrated for their softness and stretchability. The creation of glassy gels blurs this dichotomy, offering a new class of materials that are as hard and durable as glass, yet capable of stretching multiple times their original length without breaking. This paradigm shift could have profound implications across multiple industries, from electronics to biomedical devices.
What makes glassy gels particularly intriguing is their exceptional combination of strength, elasticity, and ease of manufacturing. The key lies in their unique composition: over 50% liquid, yet with structural properties that emulate solid glass. This seemingly paradoxical combination is achieved through a sophisticated chemical process involving ionic liquids—salts in ionic form—mixed with polymer precursors. When exposed to ultraviolet light, these mixtures cure into cohesive, solid-like structures. The process’s simplicity stands in stark contrast to the often complex, multi-step manufacturing procedures typical for similarly tough plastics. Such straightforward fabrication not only reduces costs but opens the door for scalable production methods, including molding and 3D printing.
Decoding the Science: How Do Glassy Gels Work?
The fundamental science behind glassy gels centers on the interaction between polymers and ionic liquids. Traditional polymers become soft or stretchable when solvents—like water—disrupt their molecular chains. However, in glassy gels, the ionic liquid’s ions strongly attract the polymer chains, restraining their movement and rendering the material glass-like in hardness. Yet, because the ionic liquid is a solvent, it also creates molecular spacing that allows the material to stretch without succumbing to brittle fracture.
This delicate balance explains why these materials defy expectations: they are stiff enough to resist breaking under considerable force, yet flexible enough to deform drastically. Furthermore, the presence of ions in the liquid enhances the material’s electrical conductivity, setting it apart from conventional plastics. Such conductivity could make glassy gels ideal for flexible electronics, wearable sensors, or energy-efficient coatings. The ability to stretch up to five times its original length and then return to the initial shape after heating is particularly noteworthy, revealing potential for reusable, resilient components.
However, what remains somewhat mysterious is the high adhesiveness of these materials. Their strong stickiness is unusual among hard, glassy substances, and the exact mechanisms are yet to be fully understood. This property may unlock novel adhesive applications where durability and reusability are vital—think biomedical adhesives, underwater bonding, or high-performance seals. The challenge here is deciphering how to manipulate these molecular interactions to optimize adhesion without compromising other desirable characteristics.
The Future Implications: Disrupting Industries and Sparking Innovation
The emergence of glassy gels is more than a scientific novelty; it heralds a potential revolution across numerous sectors. For electronics manufacturing, their high electrical conductivity and robustness could lead to new kinds of flexible devices, embodied in foldable smartphones, wearable tech, or stretchable sensors. In biomedicine, the combination of toughness, flexibility, and adhesive quality paves the way for advanced prosthetics, bio-compatible implants, or regenerative scaffolds that can endure mechanical stress while maintaining intimate contact with biological tissue.
Moreover, the ease of fabrication makes these materials especially attractive for rapid prototyping and custom applications. Unlike conventional plastics requiring extensive processing in specialized facilities, glassy gels can be cast into complex shapes using simple molds or immediately produced via 3D printing. This flexibility not only accelerates development cycles but also democratizes access to advanced materials. The potential to craft durable yet stretchable items on-demand could transform fields as diverse as aerospace, automotive, and consumer goods.
Nevertheless, skepticism is warranted. As an outsider, one must recognize that the long-term stability, environmental impact, and scalability of glassy gels still require thorough investigation. Questions about how these materials age under operational conditions or how to recycle them effectively remain unanswered. Despite these concerns, the optimism expressed by researchers underscores a belief in their transformative potential—if these hurdles are addressed, glassy gels could become a cornerstone material in the next generation of technological solutions.
The implications go beyond mere material improvement; they challenge fundamental notions about the boundaries between solidity and fluidity. Such breakthroughs serve as a reminder that innovation often arises from questioning established categories—what if the rigidity of glass and the softness of gel could coexist? The answer appears within these newly engineered glassy gels, promising a future where materials are more adaptable, durable, and functional than ever before.