The efficiency of a battery largely hinges upon the interaction between its electrodes and electrolytes. This interface plays a crucial role in energy conversion, a realization that has spurred innovative approaches to battery design in recent years. Among various strategies, researchers are particularly excited about lithium-metal batteries (LMBs), which promise to outmatch traditional lithium-ion batteries (LiBs) significantly in energy density and charging rates. By replacing graphite anodes with lithium metal, LMBs have the potential to serve a wide range of applications, from electric vehicles to renewable energy storage. Nevertheless, the practical implementation of LMBs has faced numerous obstacles that necessitate urgent and effective solutions.
Despite their allure, LMBs are not without shortcomings. Key issues include manufacturing costs, low Coulombic efficiency, and the notorious formation of lithium dendrites during the charging process. These dendrites—tree-like structures that grow on the surface of the anode—pose significant dangers such as overheating and even battery fires, while undermining overall performance. As researchers strive to cultivate safer and more reliable LMBs, understanding the regulation of lithium ion (Li+) solvation and the design of new electrolyte compositions has emerged as a primary focus. However, insights into how the dielectric environment of a battery affects this key interface remain an underexplored area of research.
Recently, a collaborative research team, led by scientists from Zhejiang University in China, made strides in investigating the role of dielectric properties in the stabilization of the electrode/electrolyte interface. Their research, published in *Nature Energy*, seeks to address the fundamental challenges that limit LMB performance. The study introduces a dielectric protocol that enhances the integrity and stability of the interfacial layer, potentially mitigating some of the pressing safety concerns associated with LMBs. According to Xiulin Fan, one of the study’s authors, improving the energy density of batteries to over 500 Wh/kg is essential for the transition to a carbon-free economy.
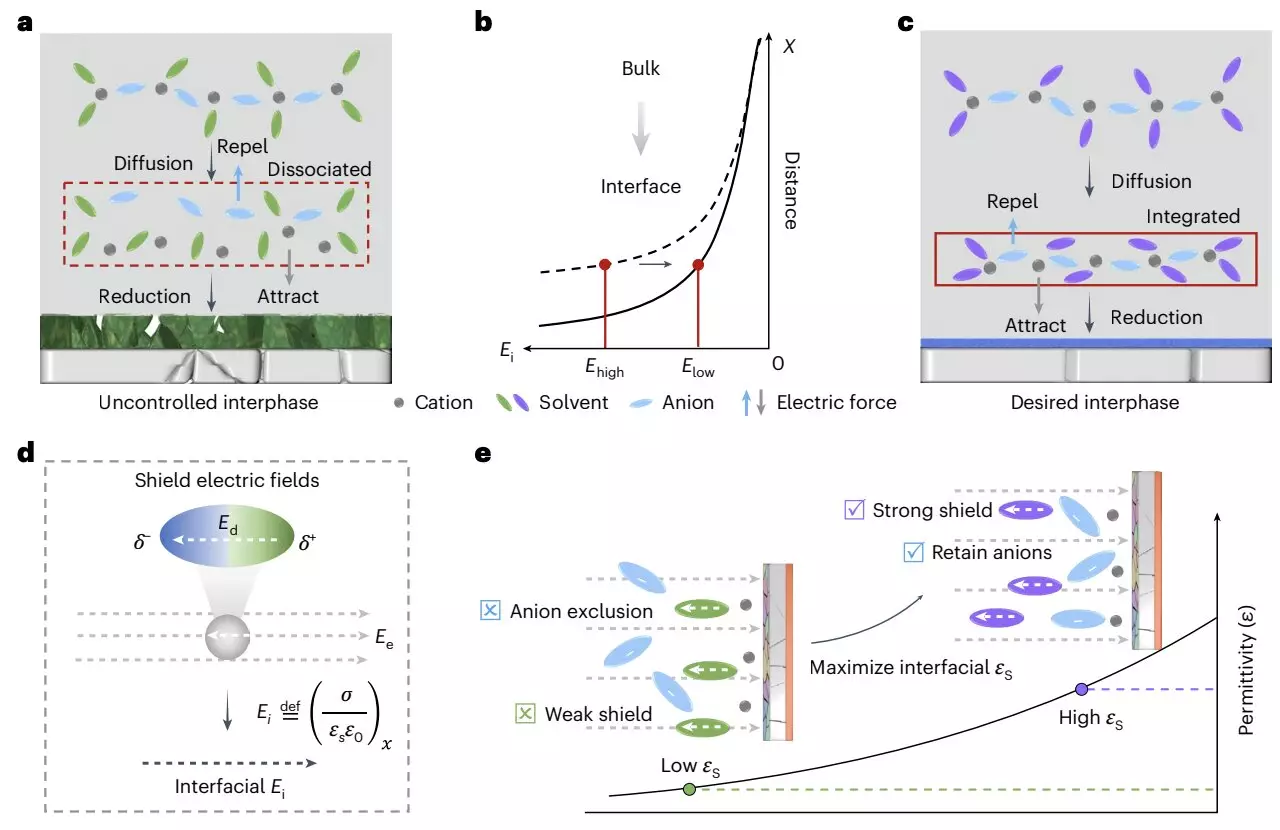
The groundbreaking findings of this research are built upon a unique conceptual framework focusing on the electric field at the interface between electrodes and electrolytes. By manipulating the dielectric environment surrounding these components, the researchers can influence cation-anion arrangements and ultimately facilitate the formation of a solid-electrolyte interphase (SEI). Their protocol requires employing a solvent with a high dielectric constant and a specific composition. This setup prevents the dissociation of cation-anion pairs, creating an anion-rich area adjacent to the electrode-electrolyte interface, thus favoring the stability of the interfacial chemistry.
Fan elaborates on how this dielectric protocol can architect a more conducive environment for lithium deposition, indicating that such a mechanism can effectively prevent the unwanted growth of lithium dendrites. The study emphasizes that a properly manipulated dielectric condition can augment the stability of the charged interface and even suppress enzyme decomposition within the assembly.
The application of this dielectric protocol resulted in the development of an ultra-lean electrolyte, which was subsequently tested in lithium-metal pouch cells. Impressively, these pouch cells achieved energy densities of up to 500 Wh/kg, reaffirming the protocol’s efficacy in enhancing battery performance. The research team’s innovative approach not only elucidates the spatial arrangement of lithium ions but also highlights the potential for tailored electrolyte compositions to serve as a means for fine-tuning interfacial properties.
The findings from this study open new avenues for research in battery technology, offering other groups a blueprint for exploring advanced electrolyte formulations that integrate similar principles. While the promise of high-energy-density LMBs is enticing, the associated safety hazards, including risks of fires and explosions, cannot be overlooked. Therefore, continued investigation into stabilizing the electrode/electrolyte interface is crucial—especially as the demand for larger-scale applications of batteries continues to surge in tandem with the rise of electric vehicles and renewable energy systems.
The journey toward optimizing lithium-metal battery performance necessitates ongoing innovation and a multifaceted understanding of electrochemical interactions. As breakthroughs like the dielectric protocol emerge, we can hope for a future where batteries are not only high-performing but also safe and reliable, paving the way for a more sustainable energy landscape.