The relentless pursuit of sustainable energy sources has prompted groundbreaking innovations in hydrogen production, with a recent study led by Prof. Chen Changlun at the Hefei Institutes of Physical Science standing out as a pivotal change agent. This research spotlights the development of cobalt-doped nickel hydroxide bipolar electrodes and non-noble metal catalysts designed to enhance the efficiency and stability of two-step water electrolysis. In an era where the implications of renewable energy adoption are urgent, these advancements not only address performance inefficiencies in traditional alkaline electrolyzers but open doors for widespread implementation in various sectors.
Challenges of Traditional Methods
Traditional alkaline electrolyzers often grapple with limitations that hinder their compatibility with fluctuating renewable energy sources. Issues such as mismatching energy supply and the potential for dangerous hydrogen/oxygen mixing under high pressure are significant barriers to their integration. The conventional systems necessitate costly membrane separators, adding to both complexity and financial burden. In contrast, the two-step water electrolysis method proposed by Changlun’s team innovatively circumvents these challenges by achieving spatial and temporal separation of hydrogen and oxygen production. This elegant solution not only reduces costs but also streamlines the process significantly.
The Science Behind the Breakthrough
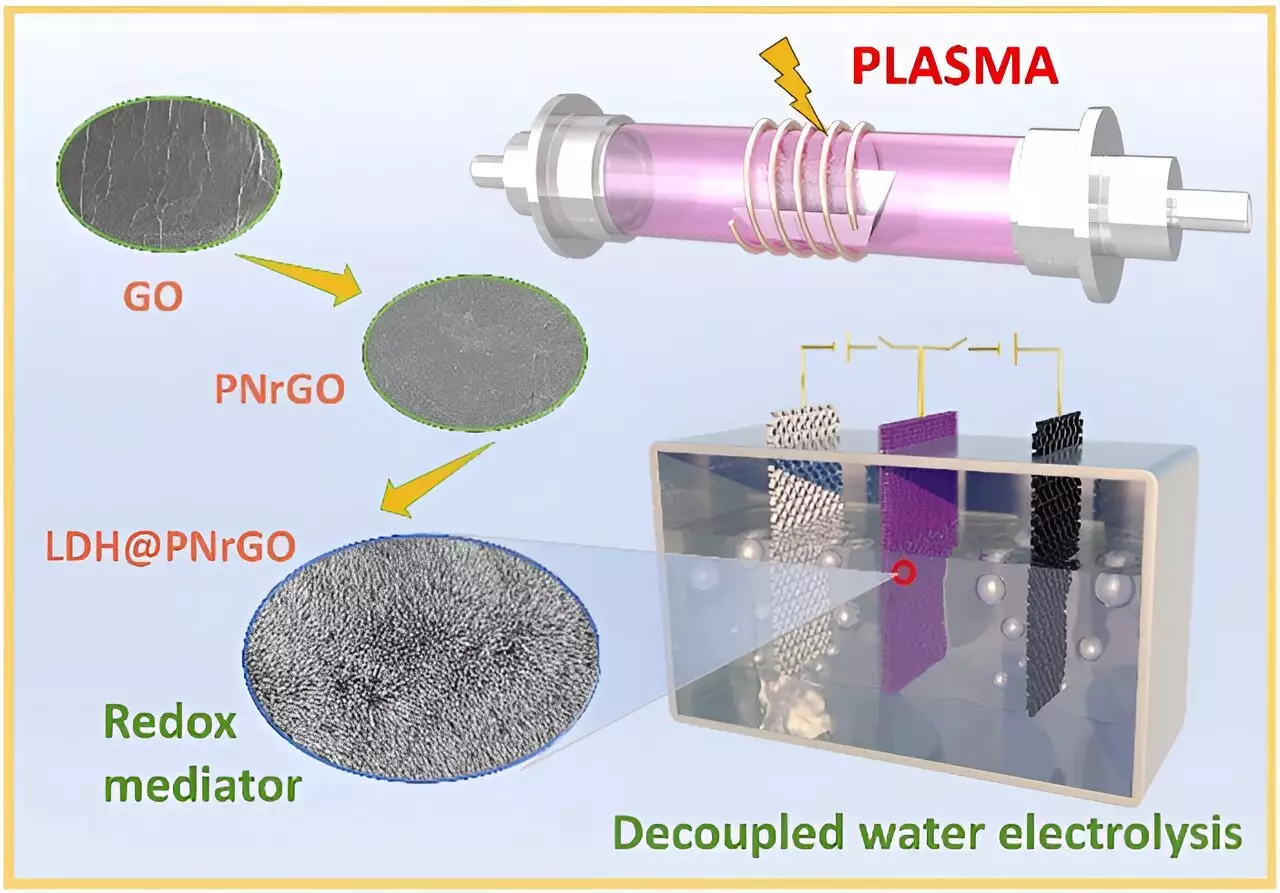
At the heart of this breakthrough lies the creation of cobalt-doped flexible nickel hydroxide bipolar electrodes through a one-step electrodeposition method. This novel approach enhances conductivity and storage performance while suppressing parasitic reactions that would otherwise waste energy through unwanted oxygen production. Furthermore, the development of non-noble metal catalysts—such as molybdenum-doped nickel-cobalt phosphide and plasma-induced iron composite cobalt oxide—reveals a commitment to sustainability without compromising efficiency. The ability of these electrodes to facilitate hydrogen and oxygen production at varied locations and times by altering current direction showcases a significant leap toward optimizing energy conversion efficiency.
Enhancing Electrode Performance with Cutting-Edge Technology
Current limitations of layered double hydroxide (LDH) electrodes, including restricted capacity and lack of stability, have been addressed through innovative techniques involving non-thermal plasma technology. The introduction of nitrogen-doped nickel-cobalt LDH and nitrogen-doped reduced graphene oxide/nickel-cobalt LDH paints a promising picture for improved electrode performance. This technological leap not only enhances electrical capacity but also positions these electrodes as ideal candidates for future large-scale hydrogen storage solutions, providing a viable option for applications ranging from 5G telecommunications infrastructure to expansive data centers.
A Vision for the Future
As the global demand for clean energy solutions significantly escalates, the advancements achieved through this research resonate as a crucial step towards practical hydrogen production applications. Prof. Chen Changlun’s assertion that their performance indicators are in sync with global advancements highlights the importance of continued innovation in this field. The promise of two-step water electrolysis not only represents an engineering triumph but symbolizes hope in realizing a future where hydrogen plays a central role in our energy landscape. In an increasingly uncertain climate, the strides made by Changlun and his team could mark the dawn of a new, sustainable energy era—one that we are all eager to embrace.