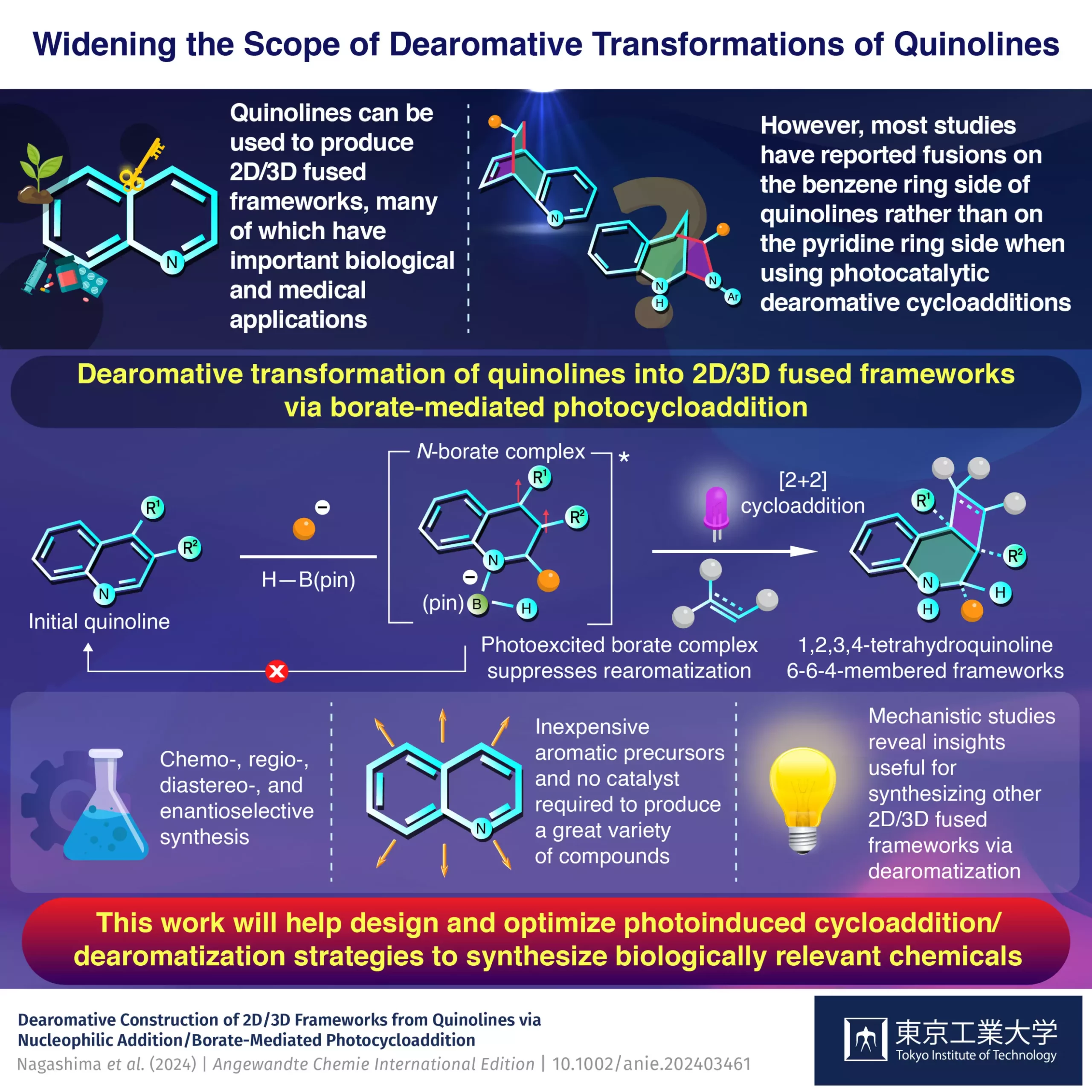
In the world of organic chemistry, the development of new strategies for synthesizing complex molecular structures is of paramount importance. Recently, a groundbreaking approach utilizing inexpensive quinolines has emerged, offering a promising method for creating 2D/3D fused frameworks. This innovative synthesis method, pioneered by a team of researchers from Tokyo Institute of Technology, highlights the potential of quinolines not just as starting materials, but as versatile compounds that could lead to customizable drug candidates with significant medical applications.
Quinolines have historically captured the interest of chemists due to their unique electronic structure, consisting of a benzene ring that is electron-rich and a pyridine ring that is electron-poor. This dual nature allows for the independent modification of these rings, making quinolines ideal candidates for complex syntheses. By employing a light-sensitive intermediate in combination with quinoline derivatives, researchers have managed to sidestep traditional barriers in synthesis, demonstrating that the potential of quinoline remains vastly underexplored.
Harnessing the Power of Light
One of the most intriguing methods identified in this research involves “dearomative photocycloaddition,” a process where light is used to destabilize an aromatic ring, thereby allowing for the attachment of various reactants. While previous studies predominantly targeted the benzene side of quinolines, this new methodology specifically addresses the less frequently explored pyridine component. This pivot not only broadens the scope of potential outcomes but also calls for a reassessment of the roles quinolines can play in organic synthesis.
What sets this research apart is the effective use of pinacolborane, a boron-containing compound, which serves as the catalyst in this groundbreaking method. The researchers found that pinacolborane facilitated the photocycloaddition predominantly on the pyridine side of the quinoline, leading to a wide array of 2D/3D frameworks with higher yields than seen in earlier methodologies. This efficiency is critical; in an age where time and cost are primary concerns in chemical manufacturing, this innovative approach could streamline the process significantly.
Unveiling the Mechanism
The underlying mechanism of this synthesis process was a focal point of the research. Assisted by organolithium compounds, the initial reaction with quinoline sets off a cascade of events leading to the formation of a stable borate complex. This borate not only accelerates the cycloaddition but also inhibits the rearomatization process, a common pitfall in conventional photocycloaddition reactions that often leads to low yields and unwanted by-products. The meticulous approach of the research team, including detailed experimental and theoretical analyses, ensures that every step is accounted for, paving the way toward greater precision in synthesis.
According to Assistant Professor Yuki Nagashima, these breakthroughs extend beyond mere theoretical implications; they open doors for incorporating boron into photocycloadditions in ways previously thought unattainable. The implications for developing organoboron compounds are profound, as they extend the toolkit available for chemists, especially in the synthesis of multi-functional compounds that can cater to an array of applications in pharmaceuticals and materials science.
The Future of Organic Synthesis
The advantages of this methodology are numerous and varied. By eliminating the need for traditional catalysts and reducing the time and steps required for synthesis, it sets a new standard in organic chemistry. The capability to utilize multi-substituted starting molecules increases the diversity of compounds that can be produced, positively impacting fields ranging from drug discovery to materials engineering.
As the scientific community continues to investigate the capabilities and applications of these newly synthesized frameworks, it is clear that the exploration of quinolines is only just beginning. The potential for creating highly customizable compounds speaks to the future of medicinal chemistry, where personalized medicine is rapidly becoming a reality. With ongoing research and development, we may soon find that the synthesis strategies pioneered by these researchers will become standard practice in laboratories around the world, marking a significant turning point in organic synthesis.