The world of chemistry continually evolves as new discoveries refine our understanding of fundamental elements. A recent study conducted by scientists at the University of Auckland is a prime example of this ongoing journey of exploration. By revisiting the properties of gallium—discovered nearly 150 years ago—researchers have revealed groundbreaking insights that challenge long-held assumptions and illuminate the metal’s unique characteristics. This investigation not only increases our understanding of gallium but also has profound implications for future technologies, including nanotechnology and materials science.
Gallium: An Element of Paradoxes
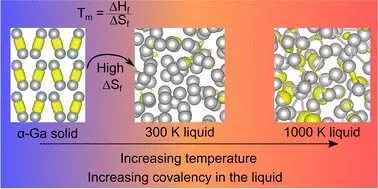
Gallium, identified by French chemist Paul-Émile Lecoq de Boisbaudran in 1875, is often recognized for its unusual melting point, which allows a gallium spoon to dissolve in hot tea. This peculiar property shapes gallium’s identity as a metalloid that defies typical metallic behavior. In contrast to most metals, gallium exists as atomic pairs called ‘dimers’ rather than single atoms, which introduces an oddity in its solid-state, rendering it less dense compared to its liquid form.
The recent findings highlight a complex, dynamic interplay between the bonds forming within gallium. Traditionally, it was believed these covalent bonds, which facilitate the sharing of electrons, simply vanished upon reaching the melting point. However, the latest research reveals that these bonds re-emerge at elevated temperatures, offering an unexpected twist to the narrative of gallium’s behavior. “Thirty years of literature on liquid gallium has had a fundamental assumption that is evidently not true,” states Professor Nicola Gaston, emphasizing the significance of these new revelations.
The Implications of New Findings
Understanding these atomic-level processes is more than just an academic exercise; it has tangible implications for developing advanced materials in fields such as nanotechnology. The researchers, including Dr. Steph Lambie and Dr. Krista Steenbergen, focused on temperature’s role in gallium’s structure, providing clues to material manipulation at the nanoscale. Gallium’s behavior is integral to innovations involving liquid metal catalysts and self-assembling structures—creating ordered materials from a previously disordered state.
Furthermore, the findings open doors to future applications, such as the potential use of gallium in creating ‘zinc snowflakes,’ an innovation developed by the same team. This research suggests that gallium could be pivotal for engineers and scientists attempting to craft new technological solutions. The disorder-to-order transition that gallium exhibits may serve as a guiding principle for designing more effective materials applicable in various industries.
A Closer Look at Gallium’s Origins and Applications
Interestingly, gallium’s presence in nature diverges from that of many other metals. It is not found in its pure form in nature; rather, it is extracted from ores and minerals like bauxite. This links gallium to several modern technological marvels, as it plays a crucial role in fabricating semiconductors, telecommunication systems, and more. Its applications extend to light-emitting diodes (LEDs), laser diodes, and solar panels, making it a cornerstone of contemporary electronics.
Moreover, the metal’s unique characteristics position it as a valuable asset in the quest for extraterrestrial life. Researchers investigating Mars have contemplated gallium’s potential as a chemical ‘fingerprint’ that may provide evidence of past microbial existence. Such interdisciplinary research initiatives illustrate gallium’s significance beyond traditional boundaries, merging the realms of chemistry, environmental science, and space exploration.
The Legacy of a Timeless Discovery
The historical backdrop of gallium enriches its narrative. Dmitri Mendeleev, the revered Russian chemist, famously predicted gallium’s existence before it was discovered, highlighting the foresight that characterized his creation of the periodic table. Gallium’s nomenclature pays homage to Gaul—now modern-day France—and signifies a geographical connection crucial to its discovery.
As we stand on the precipice of advancements fueled by this updated knowledge of gallium, its story encapsulates the spirit of scientific inquiry—one that constantly seeks to challenge conventions and embrace new interpretations. The evolution of gallium from a simple metalloid to a pivotal element in emerging technologies exemplifies not only the importance of its characteristics but the constant need for scrutiny and re-examination in the pursuit of scientific understanding. Each discovery paves the way for new applications, reiterating that the journey of knowledge is ongoing, and the secrets of elements like gallium still hold untold possibilities.