The allure of fragrances has captivated humanity since the dawn of civilization. Throughout history, pleasant aromas have been intertwined with notions of beauty, health, and even spirituality. The quest for an enchanting scent has led to the creation of perfumes that not only enhance allure but also evoke powerful emotions. One of the most celebrated and elusive fragrances in the world is ambrox, a compound that has long been derived from the natural secretion known as ambergris, originating in the digestive system of sperm whales. However, recent advancements in chemistry suggest a promising shift that could radically change how ambrox is sourced, echoing a growing trend in sustainable science.
The historical extraction of ambrox from ambergris has always posed ethical and environmental dilemmas. With only about 30 tons being harvested annually, the process is not just ecologically sensitive but also fraught with unpredictability due to the limited supply of ambergris. Attaining this prized scent from marine mammals has spurred debates regarding conservation and the future of fragrance production. While fetching this natural resource might evoke a sense of nostalgia for traditional perfumery, it raises essential questions about sustainability and ethical sourcing.
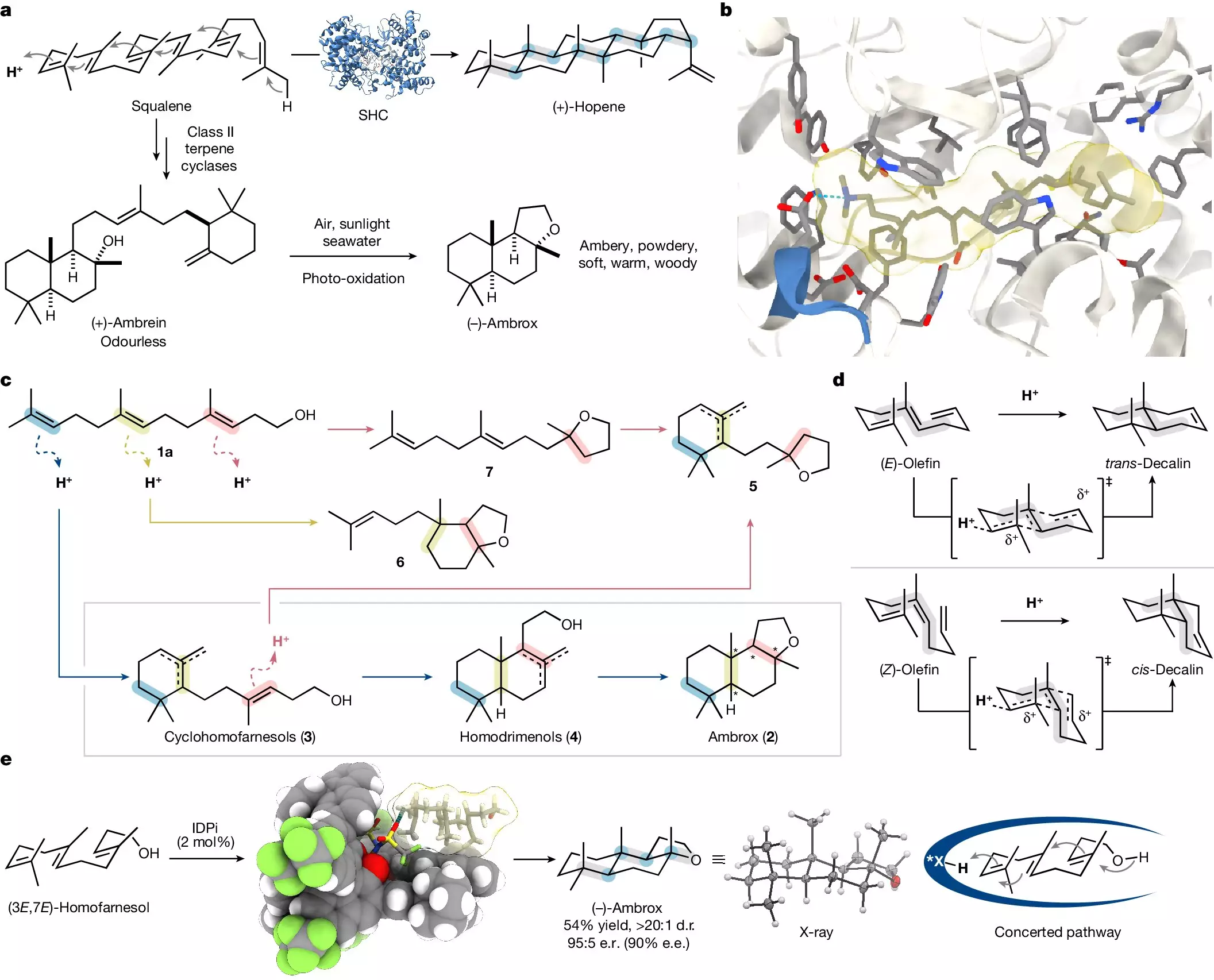
Fortunately, scientists have paved new paths in fragrance synthesis. One significant advancement is the partial synthesis derived from clary sage, a plant abundant in nature. However, this process is contingent upon the availability of clary sage, which can fluctuate. The challenges surrounding both natural and semi-synthetic methods led to the pressing need for a more efficient and reliable alternative.
Led by Professor Benjamin List at the Max Planck Institute for Coal Research, a new method has emerged that revolutionizes how ambrox is synthesized. In a remarkable feat published in the journal *Nature*, the research team unveiled a sophisticated approach using lab-based techniques to produce this coveted fragrance. Their method diverges from traditional practices and leans heavily on innovative catalytic processes.
At the core of this revolutionary synthesis is the catalytic asymmetric polyene cyclization, a state-of-the-art technique that demonstrates how simple molecular building blocks can converge to form complex structures. Mathias Turberg, a doctoral student and co-author of the study, likens their inspiration to nature itself, highlighting how this approach mirrors the intricate processes that occur in biological systems.
Dr. Na Luo, another driving force behind the research, emphasizes that this methodology reflects an ongoing challenge for chemists: mimicking nature’s complexity in a laboratory setting. Such a goal not only advances the field of synthetic chemistry but also aligns closely with the ethical imperatives of modern science, aiming to reduce our ecological footprint.
The research team utilized nerolidol—an essential building block found in many plants—as their starting material. Collaborating with the chemical company BASF, they transformed nerolidol into homofarnesol, subsequently leading to the synthesis of (-)-ambrox. The crux of their work lies in the utilization of a tailored catalyst within a specifically chosen fluorinated solvent. This combination allows them to selectively produce the desired chiral isomer from an array of potential variations.
In a notable comparison to existing biocatalytic methods, which typically extend over several days, List and his team demonstrate that their procedure can yield ambrox overnight. This not only highlights the efficiency of their method but also suggests scalable applications that could shift the industry’s production paradigm ultimately.
Moreover, a key aspect of their research is the recoverability and recyclability of both the catalysts and solvents used, presenting an added layer of sustainability critical for future industrial applications. The prospect of synthesizing complex fragrance compounds in a single step with minimal environmental impact is a notable leap forward in the chemistry community.
The innovative work by List’s group on the synthesis of ambrox signifies more than just a scientific advancement; it marks a transformation in the fragrance industry. As we continue to explore and innovate within the realm of chemistry, the possibilities for creating sustainable and ethically sourced fragrances appear more tangible than ever, holding the potential to redefine our relationship with scent.