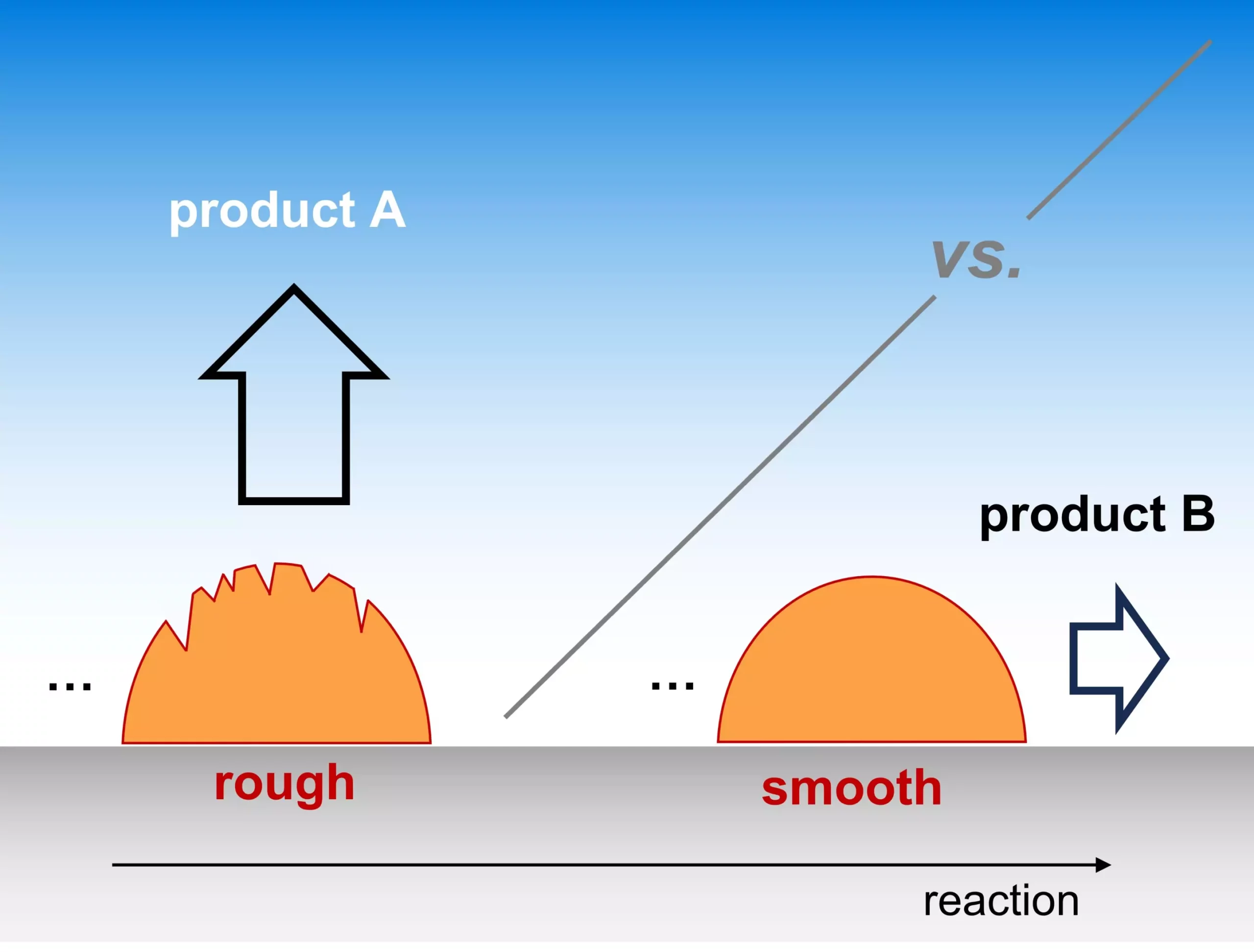
Recently, the Theory Department of the Fritz Haber Institute shed light on an underappreciated aspect of electrocatalysis: catalyst morphology. Instead of simply focusing on the atomic characteristics of active sites, the team emphasizes the significance of the surface roughness of catalysts, which profoundly impacts the selectivity of various reactions. This new insight can redefine our approach to designing catalysts, particularly in technologies critical for sustainable energy production, such as converting CO2 into usable fuels.
Redefining Selectivity: A Shift in Perspective
The conventional understanding of catalyst efficiency predominantly revolves around the properties of the active sites; however, this research introduces a paradigm shift. The authors argue that the roughness of a catalyst’s surface can dictate which products are formed during a reaction. This notion is particularly critical in electrocatalytic contexts, such as in fuel cells, where stable operation is crucial. By investigating how microscopic features on catalyst surfaces influence reaction pathways, the team has opened the door to optimizing catalysts with a newfound focus on morphology.
Understanding Heterogeneous Electrocatalysis
Heterogeneous electrocatalysis is a linchpin in sustainable energy technologies, allowing for the carbon-neutral generation of fuels and chemicals directly from renewable electricity. The processes involved often occur at mild temperatures and pressures, driven primarily by charge transfer mechanisms at the solid-liquid interface. The implications of the Fritz Haber Institute’s findings transcend theoretical applications and touch on real-world issues, such as climate change and the quest for alternative energy sources.
A New Kinetic Model: Bridging Theory and Practical Application
The researchers have developed a multi-scale kinetic model that encapsulates how catalytic selectivity hinges on the transport rates of species in the electrolyte, alongside a quantifiable effect stemming from catalyst roughness. Remarkably, this model is not just an abstract concept; it aligns closely with a range of experimental observations, solidifying its validity. This ability to reproduce empirical trends underscores the transformative potential of focusing on surface morphology in catalyst design.
Expanding the Horizon: Future Directions in Catalyst Design
What this study highlights is more than just a new facet of catalyst design; it establishes roughness as a critical variable influencing the efficiency of electrocatalysts across various processes. This prompts a radical reevaluation of how future catalysts should be structured, ensuring that designers consider morphological features alongside the traditional concerns regarding atomic structure. By advancing our understanding of reaction mechanisms in electrocatalysis, this research paves the way for unraveling complex interactions within systems, ultimately leading to innovations that could support the creation of more efficient energy technologies.
In a world increasingly focused on sustainable solutions, this breakthrough stands out. Significantly, it reiterates that the nuances of catalyst morphology are not merely technical details; they are fundamental elements that can drive meaningful progress in meeting global energy challenges.