In recent years, vaping has gained popularity as a perceived safer alternative to traditional smoking, often marketed with claims of reduced health risks. However, emerging scientific evidence suggests that the fundamental ingredients in vape liquids—propylene glycol and glycerol—may themselves pose significant health concerns, especially during pregnancy. A groundbreaking study conducted by researchers at Ohio State University reveals that even nicotine-free vape formulations can have adverse developmental effects on offspring, challenging the misconception that avoiding nicotine guarantees safety. This insight forces us to reconsider the narrative surrounding “safe” vaping, particularly for vulnerable populations such as pregnant women.
Dissecting the Ingredients: More Than Just Carriers
Vape liquids typically consist of two main carrier substances: propylene glycol and glycerol (commonly known as vegetable glycerine). These substances are intended to carry flavors and nicotine, but their intrinsic biological impacts are often overlooked. The recent study meticulously isolated these carrier fluids, creating two formulations with different ratios—50/50 and 30/70—without any added nicotine or flavorings. The goal was to establish a clear baseline of how these base ingredients interact with biological systems, independent of other chemicals.
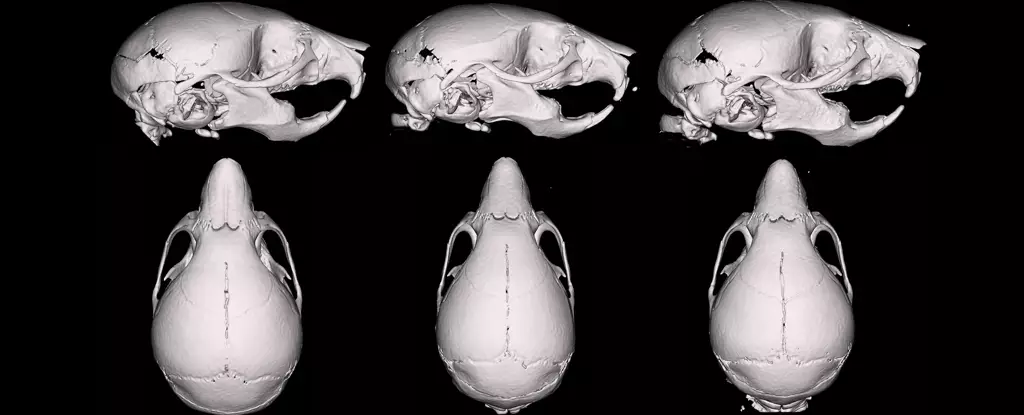
What the researchers found was startling. Exposure to a higher glycerol ratio (30/70) during pregnancy resulted in offspring with notably smaller skulls and narrower facial structures. This was counterintuitive, considering the initial assumption that the mixture with more propylene glycol (50/50) would be more harmful, given prior associations of propylene glycol with increased nicotine uptake and potential toxicity. Instead, the lower propylene glycol mixture caused more pronounced developmental changes, indicating that glycerol, once considered inert, may have underappreciated biological effects.
Implications for Fetal Development and Public Health
The reduction in skull and face size among pups exposed to the 30/70 mixture suggests that these carrier fluids can influence craniofacial growth and overall development, even in the absence of nicotine. These morphological changes mirror some developmental patterns observed in human children with certain growth deficiencies, raising alarm about the broader implications of inhaling these substances during pregnancy.
The study’s findings imply that the notion of “safer” vaping because of nicotine avoidance is fundamentally flawed. Pregnant individuals might assume that avoiding nicotine shields their unborn children from harm, but the research indicates that the very foundation of many vape products—propylene glycol and glycerol—may have intrinsic developmental risks. Moreover, the observed decrease in body weight, albeit within normal ranges, adds to the growing concern that these substances may subtly disrupt normal growth patterns.
This realization comes at a crucial time when vaping is often heavily marketed as a benign habit. For pregnant women or those planning to conceive, this research underscores the importance of reevaluating the safety of any aerosolized substances ingested or inhaled during gestation. The possibility that fundamental carrier ingredients could interfere with fetal development necessitates immediate attention and cautious behavior.
Challenges in Assessing the True Risk to Humans
While animal studies like this provide valuable insights, they are not without limitations. Ethical considerations prevent direct experimentation on humans, and observational studies on vapers face confounding variables and long latency periods. Additionally, variability in the composition of commercial vape products means that what is tested in the lab may not perfectly reflect real-world use. Nonetheless, these findings are alarming because they signify that even in the absence of nicotine and flavorings, the primary carrier substances are not as benign as once believed.
Given that vaping’s appeal is largely based on perceptions of reduced harm, this research pushes us to scrutinize the very foundational ingredients. If propylene glycol and glycerol can influence developmental outcomes in mice, the risk to human pregnancies—especially with chronic exposure—may be even more significant. This mandates a cautious approach and increased regulatory oversight.
The Path Forward: Rethinking Safety and Regulation
As the public health community grapples with these revelations, it becomes imperative to deepen our understanding of vaping’s true risks. Future research should focus on long-term human outcomes, but in the meantime, healthcare professionals and policymakers must advocate for heightened awareness about the potential dangers of even nicotine-free vaping.
Manufacturers should be compelled to disclose exact contents and undergo rigorous safety testing of carrier ingredients. Pregnant individuals should be advised to abstain from any form of inhalation involving these substances until clear safety profiles are established. The assumption that vaping—especially nicotine-free variants—is harmless is a perilous misconception that this study effectively dismantles.
Ultimately, this research challenges the complacency that has often surrounded the vaping industry. It invites us to think critically about what’s truly safe and encourages a shift toward more informed choices, particularly for those most vulnerable to developmental disturbances. The foundational ingredients in vape products are not just inert carriers; they may hold significant biological influence—and that influence appears to be far from benign.