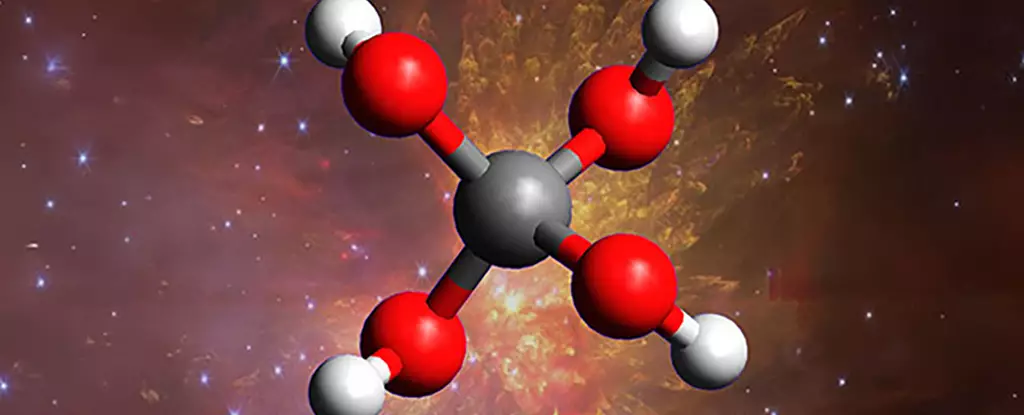
For centuries, humanity has looked up at the night sky with curiosity and wonder, pondering what mysterious molecules and compounds might exist among the stars. Recent experimental breakthroughs push these questions further, challenging our understanding of space chemistry. In an ambitious effort to simulate the extreme conditions of interstellar clouds, scientists have managed to produce a molecule long theorized but never before observed: methanetetrol, a ‘super alcohol’ composed of four hydroxyl groups attached to a single carbon atom. This discovery is a testament to human ingenuity and pushes the boundaries of what we believe is chemically possible in the cold depths of space.
This achievement is not merely about confirming a theoretical prediction; it represents a fundamental shift in our perception of cosmic chemistry. Traditionally, space was viewed as a barren expanse with simple molecules, but the synthesis of methanetetrol reveals a far more complex and dynamic environment. By replicating the conditions of deep space—ultra-cold temperatures, a vacuum, and radiation bombardment—researchers have demonstrated that the universe can host molecules that defy Earth’s familiar chemistry. Such findings compel us to reconsider the diversity and richness of interstellar molecules and their potential role in the cosmos.
Scientific Ingenuity in Simulating Space Conditions
Creating methanetetrol in a laboratory setting was no small feat. To mimic the extraterrestrial environment, scientists froze a mixture of carbon dioxide and water, forming an icy substrate akin to space dust and ice conglomerates. Exposing this simulated “space ice” to cosmic-ray-like radiation initiated complex chemical reactions that eventually led to the formation of methanetetrol. The process highlighted the surprising capability of high-energy radiation to catalyze intricate organic synthesis in extreme conditions previously thought to be inert.
What makes this breakthrough particularly compelling is the molecule’s instability. Methanetetrol is so reactive that it rapidly disintegrates when exposed to light—a process called dissociative photoionization—making it exceptionally challenging to detect or study in natural space environments. Therefore, the laboratory’s success hinges on the transient observation of this molecule, which suggests that it exists fleetingly amidst interstellar clouds, possibly contributing to prebiotic chemistry. This fleeting existence, however, does not diminish its significance; instead, it underscores how space’s harsh, energetic environment can forge complex molecules in ways terrestrial labs cannot easily replicate or observe.
The Implications: Unlocking Mysteries of Space and the Origins of Life
The discovery of methanetetrol opens up a spectrum of possibilities for scientists probing the universe’s chemical makeup. If such a ‘super alcohol’ can be synthesized in the icy depths of space, it begs the question: what other complex, unstable molecules are out there lurking unseen? These molecules could serve as precursors or catalysts in the complex chain of reactions leading to the formation of life or other biologically relevant compounds. They suggest that the interstellar medium might be a fertile ground for organic synthesis, far more intricate than previously believed.
Moreover, this discovery might influence models of star and planet formation. Organic molecules like methanetetrol could play a role in the chemistry of protoplanetary disks, influencing the initial chemical inventory of nascent planets. It raises the exciting possibility that certain molecules essential for life might form naturally in space, ready to be delivered via cometary impacts or dust infall—a cosmic chemistry kit preparing the stage for life’s emergence elsewhere.
Still, practical challenges remain. Detecting methanetetrol in actual space environments will be arduous due to its fragile nature. The molecule’s quick dissociation when exposed to light hampers traditional observational methods. Yet, with advancing telescope technologies and spectroscopic techniques, astronomers are now better equipped to hunt for indirect signatures or precursors, bringing us closer to confirming these molecules’ cosmic presence. Each breakthrough expands the frontier of astrochemistry, transforming our understanding of how reality in the universe surpasses even our most ambitious theories.
Looking Ahead: A Future Filled with Boundless Chemical Discoveries
The synthesis and detection of methanetetrol mark a significant milestone, yet it is only the beginning. As researchers explore the limits of space chemistry further, new, more exotic molecules are likely to emerge from labs and telescopic observations alike. The universe’s chemical diversity appears to be far richer than we’ve imagined, echoing a cosmic symphony of reactions that challenge our earthly assumptions.
This revelation invites us to think beyond the traditional boundaries of chemistry. The universe might be a colossal laboratory, continuously experimenting with molecules too unstable or complex to sustain on Earth. By studying these phenomena, we’re not just uncovering the building blocks of space but also gaining insights into the potential pathways for life’s origin elsewhere in the cosmos. As the scientific community continues to push into these uncharted territories, we inch closer to answering age-old questions about our place in the universe and the myriad forms of chemistry that might exist beyond our planetary confines.