High entropy oxides (HEOs) are emerging as revolutionary materials with potential applications ranging from electronics to energy storage. Recent research sheds light on how various synthesis techniques can significantly influence the properties of these materials. This breakthrough study, published in the Journal of the American Chemical Society, offers a deeper understanding of the enhancements in the structural and functional attributes of HEOs when synthesized using different methods.
High entropy oxides consist of an intricate blend of at least five transition metal oxides, exhibiting unique structural properties due to their diverse chemical composition. Such diversity allows for tremendous flexibility, enabling researchers to fine-tune the materials for specific applications due to their unique electrochemical characteristics. Alannah Hallas, a materials scientist at the University of British Columbia, highlights the excitement surrounding HEOs as they hold promise for next-generation electronic devices and energy systems. The versatility with which these materials can be created offers researchers a myriad of optimization parameters, paving the path for groundbreaking technological advancements.
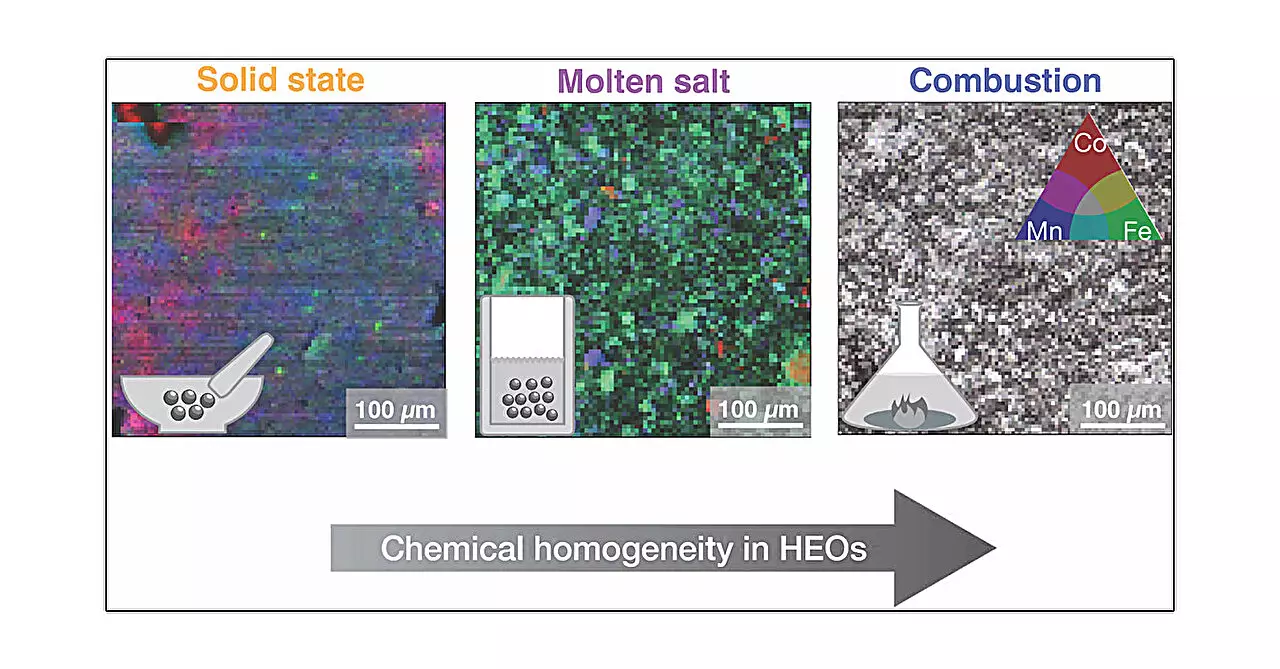
This study involved rigorous analysis of samples synthesized through five distinct methods: solid-state, high-pressure, hydrothermal, molten salt, and combustion. Each technique varies in its approach to heating and cooling the materials, thus affecting the resulting crystalline structures. The solid-state method is akin to baking, wherein metal oxides are blended and heated. In contrast, the high-pressure method simulates conditions found deep within the Earth, introducing external pressure during synthesis, which can dramatically impact crystal formation.
The hydrothermal technique employs a pressurized vessel where metal salts and water interact, encouraging crystal growth under high temperatures. Meanwhile, molten salt synthesis allows metal salts to form a viscous liquid, fostering crystal precipitation as it cools. Lastly, combustion synthesis drives the creation of HEOs through an exothermic reaction, igniting a gel-like mixture of metal salts in solution, resulting in a rapid formation of the desired material.
As lead author Mario Ulises Gonzáles-Rivas elucidates, the driving mechanisms inherent in these methods lead to varying degrees of structural and microstructural homogeneity. The combustion method, for example, produced notably homogeneous samples, showcasing that the synthesis technique can influence not just morphology, but potentially the material’s overall function.
The study’s findings elucidate that while the average bulky structure of the materials remained unchanged across the different techniques, the local and microstructural characteristics exhibited substantial variability. This discrepancy is critical, as it implies that the synergetic effects of ion mixing, crystallinity, and defects foster unique properties that can enhance performance in practical applications.
Gonzáles-Rivas notes that these subtle structural differences affect how HEOs perform in various environments, especially in applications related to energy storage and conversion. For instance, the ability to harness the favorable electrochemical properties that these materials exhibit could be compromised if the synthesis method does not align with the desired outcome.
This groundbreaking research resulted from a collaborative effort spearheaded by Hallas’ team at UBC, in conjunction with experts from the University of Saskatchewan and the Max Planck Institute for Solid State Research. By elucidating how synthesis techniques contribute to the nuanced properties of high entropy oxides, this study presents a new optimization trajectory for future research and development.
The implications of these findings extend well beyond academia; they have the potential to influence the practical deployment of HEOs in various technologies, particularly those aimed at addressing energy challenges. As the quest for efficient energy systems intensifies, understanding the significant role of synthesis methods in tailoring material properties becomes a crucial element in the innovative journey towards effective energy solutions.
This study marks a significant advancement in the field of materials science, reflecting the importance of synthesis strategies in determining the applicability of high entropy oxides for tomorrow’s technological endeavors.