Electrocatalysis plays a vital role in facilitating critical chemical reactions that are essential for energy conversion and storage technologies, such as water splitting, where hydrogen production is central to renewable energy solutions. A primary challenge in this field has been developing catalysts that are not only efficient but also stable over prolonged periods, especially under harsh conditions, such as acidic environments. The recent research surrounding erbium-doped cobalt oxide (Co3O4) presents a promising advancement, offering a cost-effective alternative to traditional noble metal catalysts.
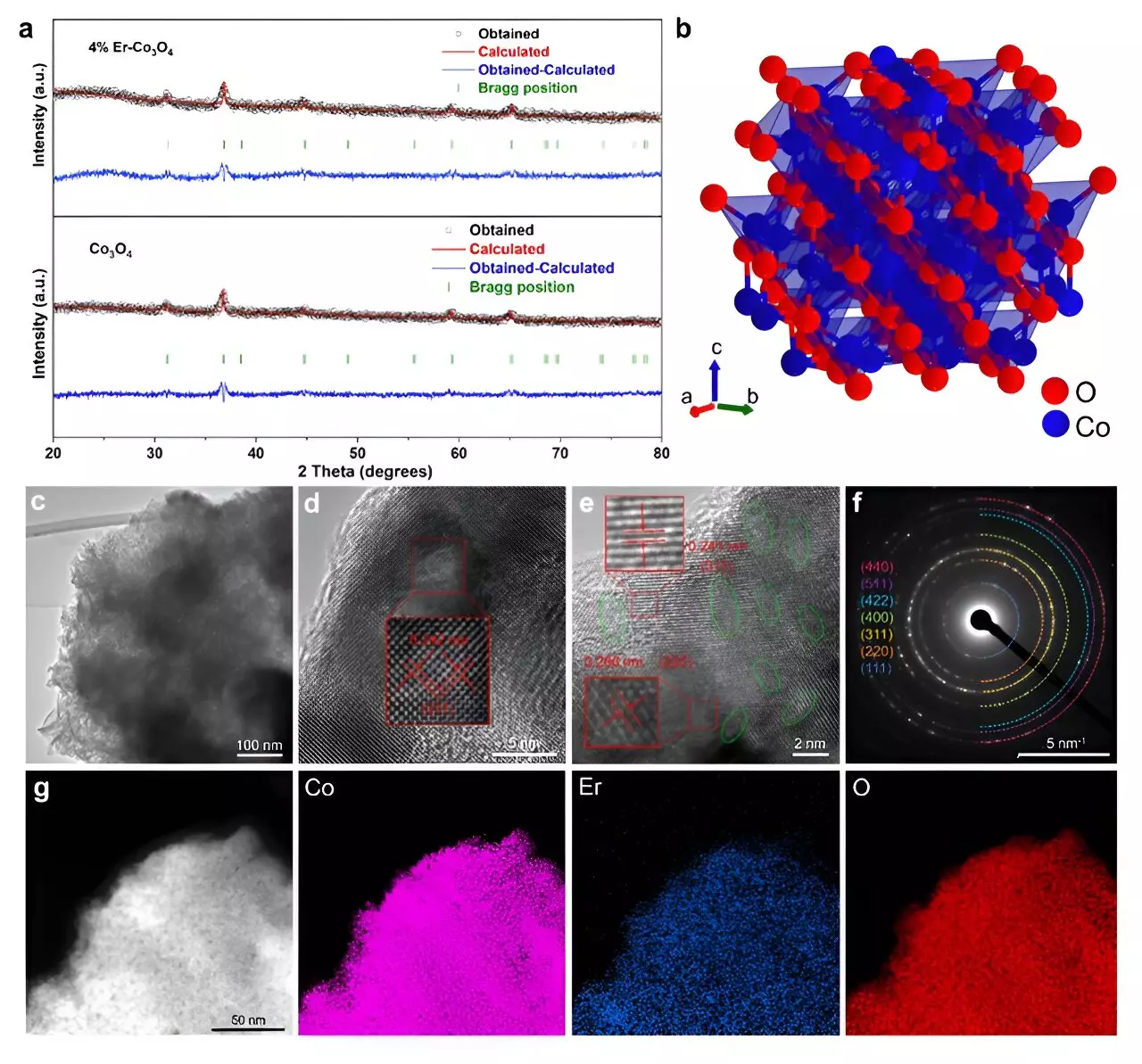
A team of researchers has unveiled groundbreaking findings related to the enhancement of OER processes by incorporating erbium, a rare earth element, into cobalt oxide catalysts. Published in the prestigious journal ACS Catalysis and recognized as an Editors’ Choice, the research demonstrates that a modest insertion of erbium into the Co3O4 structure significantly improves both efficiency and stability. The findings indicate that the 4% doped Co3O4 catalyst exhibited an impressive overpotential of 321 mV at 10 mA cm², outperforming many existing non-precious metal catalysts and even matching the efficacy of some expensive options such as iridium-based catalysts.
The team’s investigation employed advanced techniques, including microkinetic modeling and density functional theory (DFT) calculations, to elucidate the mechanisms responsible for the improved catalytic performance observed in the erbium-doped catalyst. The incorporation of erbium facilitates the creation of numerous active sites and defects within the crystal structure of Co3O4. This modification not only increases the density of Co3+ ions relative to Co2+ ions but also promotes the generation of oxygen vacancies, which are essential for accelerating the oxygen evolution reaction.
As highlighted by researchers, the analogy of the catalyst functioning like a road system can significantly clarify the underlying principles. The presence of erbium creates additional pathways—akin to extra traffic lanes—that allow more oxygen intermediates to traverse efficiently during the reaction. This increased flow of intermediates directly correlates to the catalyst’s enhanced effectiveness in facilitating the OER process.
In situ Raman spectroscopy provided further insights into the structural transformations within the erbium-doped Co3O4. The technique revealed that oxygen vacancies formed within the octahedral sites of the crystalline structure significantly influenced the development of crucial reaction intermediates. With a higher Co3+/Co2+ ratio, the catalyst’s activity is bolstered as it provides more abundant Co3+ sites capable of anchoring intermediates vital for the reaction pathway. The intricate interplay of electronic and structural optimization showcases the complexity of this catalyst system and underscores the significance of such features in achieving high performance.
These research findings are more than just incremental improvements; they represent a paradigm shift in the design of electrocatalysts that prioritize both performance and cost-effectiveness. The successful integration of a rare earth element like erbium into a transition metal oxide opens avenues for exploring other non-precious metal doping strategies. The researchers are set to further probe into the formulation of similar low-cost electrocatalysts, which could lead to significant advancements in sustainable energy production technologies.
Moreover, as the global community increasingly seeks alternatives to rare and expensive materials in technological applications, this work underscores the potential for metal doping to pave the way for both economic and environmental sustainability in fuel cell and electrolysis technologies.
The research on erbium-doped cobalt oxide catalysis marks a crucial step towards developing high-performance, low-cost catalysts suitable for energy applications. By leveraging unique metal combinations and innovative approaches to catalyst design, this work not only enhances our understanding of electrocatalytic processes but also propels us closer to sustainable energy solutions. The journey towards efficient energy conversion is intricate, but with continued exploration and research, breakthroughs like these signal promising advancements for future energy technologies.