Water resources are essential for life, but pollution from heavy metals such as cadmium and lead poses significant health risks to both humans and aquatic ecosystems. These metals can infiltrate drinking water sources, leading to alarming levels of toxicity. Conventional water purification methods, including filtration and membrane technologies, often fall short due to their energy demands and the quick clogging of filter systems. With the growing concerns around heavy metal contamination, there is an urgent need for more effective and sustainable remediation technologies.
Recent research has explored the potential of utilizing plant-derived materials for detoxifying polluted water. Polysaccharides, which are carbohydrate polymers composed of repeating sugar units, serve as natural barriers in plants that can capture heavy metal ions. For instance, earlier studies demonstrated the efficacy of polysaccharide extracts from plants like okra and aloe vera in trapping microplastics from wastewater. However, many of these polysaccharides have limitations; they dissolve in water, necessitating the addition of other agents to create insoluble forms capable of binding heavy metals.
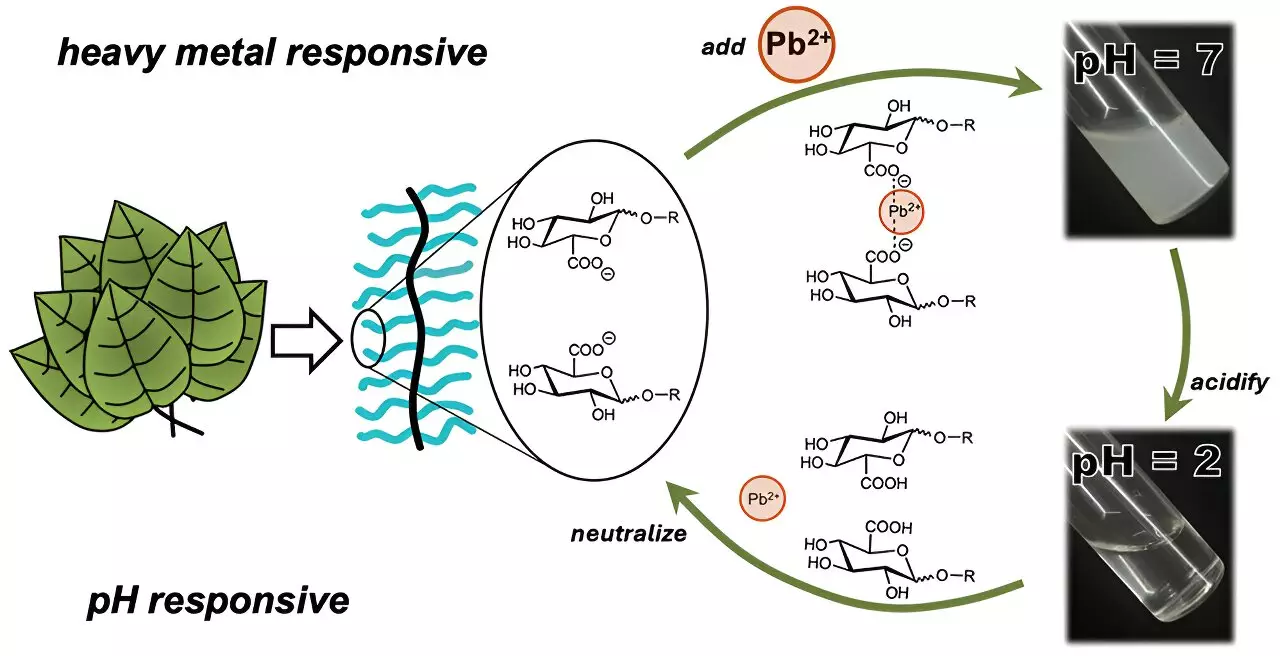
In pursuit of a more effective solution, researchers from the University of Texas at Austin focused on engineering a single material that would incorporate sugar-like structures with adjustable water solubility, allowing for enhanced removal of heavy metals from aquatic environments.
Cassandra Callmann and her team embarked on the ambitious task of designing several novel polymers that feature a water-insoluble framework supplemented by various water-soluble carbohydrate ‘charms.’ This unique design allows the polymer not only to attract heavy metals but also to facilitate their removal. In their initial experimentation, the polymer demonstrating the strongest affinity for ionic cadmium included a carboxylic acid group as part of its structure.
The ability to form visible clumps at an astonishing speed of three minutes after being introduced to cadmium-laden water showcases the polymer’s effectiveness. These clumps can easily be filtered out, presenting a significant convenience in the purification process. However, the real ingenuity emerged when adjusting the acidity of the water allowed for the clumps to dissolve back, releasing the trapped cadmium without damaging the polymer’s stability, thus proving its recyclability across multiple cycles.
To validate the practical application of their invention, the research team tested the carbohydrate-enriched polymer on water samples from the Colorado River, which had been artificially spiked with ionic cadmium and lead. The samples contained a higher concentration of other metal ions, such as calcium, sodium, and magnesium, compared to the introduced contaminants. Remarkably, over a testing span of 24 hours, the polymer was able to sequester up to 20% of cadmium and an impressive 45% of lead from the contaminated water, while only minimal amounts of the background ions were captured.
The results present a promising trajectory towards the development of reusable, selective materials designed specifically for heavy metal removal. The efficiency of this innovative polymer could drastically improve strategies for flushing out heavy metals from contaminated water, diminishing the dependence on resource-intensive methods currently in place.
The emergence of sugar-derived polymers engineered to capture heavy metals signifies a noteworthy advancement in water purification technology. By harnessing the natural affinity of plant polysaccharides for toxic metal ions and transforming them into a recyclable material with controlled water solubility, researchers are laying the groundwork for more sustainable and efficient water treatment solutions. As the crisis of water contamination persists globally, innovations like this polymer could represent the future of clean water initiatives, safeguarding ecosystems and public health alike. The ongoing research and potential enhancements in this field can contribute not only to the removal of heavy metals but also encourage the exploration of further applications related to environmental remediation.