The field of biotechnology continually pushes the boundaries of how we understand and manipulate biological systems, particularly through innovations in drug therapies and cellular treatments. A significant challenge in this endeavor has been the effective observation of biomolecules within living cells. Traditional techniques often suffer from limitations imposed by the cell’s aqueous environment. Recognizing the urgent need to enhance imaging capabilities, a team of researchers at the National Institute of Standards and Technology (NIST) has unveiled a groundbreaking method that employs infrared (IR) light to visualize biomolecules without the interference of water.
Infrared light, lying just beyond the red spectrum visible to the eye, has long been hampered in biomedical applications due to water’s robust absorption properties. Water’s predominance in cells creates a daunting challenge when attempting to detect the signal of other biomolecules, such as proteins. In a simple analogy, if you have an airplane flying overhead on a bright sunny day, it becomes nearly impossible to see the airplane without some means to filter out the sun’s glare. In this scenario, the sun represents water absorption, overwhelming the signals produced by biomolecules.
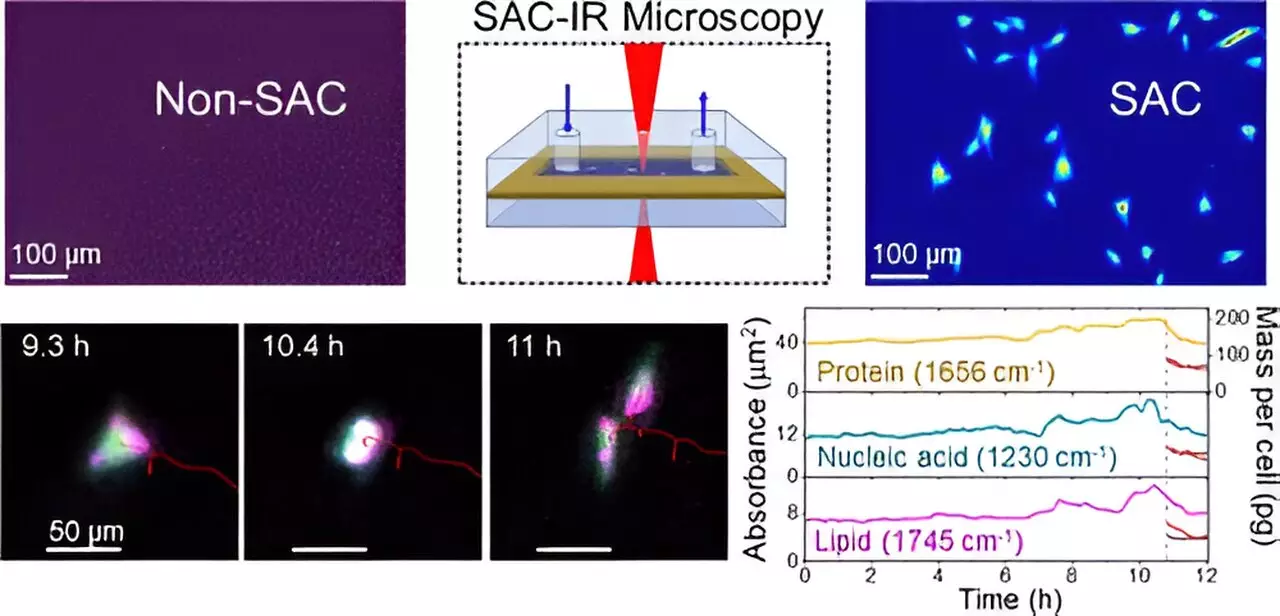
NIST chemist Young Jong Lee and his team tackled this issue head-on. Their innovative approach involves an optical strategy called solvent absorption compensation (SAC), which neutralizes the water’s masking influence in IR measurements. This advancement enables researchers to obtain clearer spectra of proteins and other cellular components, facilitating unprecedented insights into cellular function.
The feature of the SAC technique lies in its ability to render IR microscopy practical notwithstanding the interference from water. By employing a custom-built IR laser microscope, researchers can image key biomolecules contained within fibroblast cells—cells essential for connective tissue formation—across various phases of cell division over an extended observation period. This ability to capture dynamic biomolecular changes represents a significant advancement over existing methodologies that require extensive beam time and resources at large synchrotron facilities.
Moreover, the SAC-IR technique is entirely label-free. Unlike traditional approaches that rely heavily on dyes or fluorescent markers—which can potentially disrupt cellular health and complicate inter-laboratory uniformity—this method allows for direct assessment of biomolecule mass within the cells. The implications of this label-free approach are profound, offering a pathway towards standardized measurement practices beneficial across diverse fields of biology and medicine.
The ramifications of the SAC-IR method are extensive, with applications stretching from cancer cell therapy to drug discovery. For instance, in cellular immunotherapy, the ability to analyze and quantify changes in biomolecules within genetically modified immune cells could lead to better assessment protocols for their safety and efficacy. This insight is critical in ensuring that therapies deployed in clinical settings are both effective and do not inadvertently harm patients.
In the realm of drug discovery, the SAC-IR methodology opens up new avenues for screening potential drug candidates. By determining the absolute concentrations of various biomolecules in numerous individual cells, researchers can glean insights into how these cells react to new compounds. Understanding cellular responses at this granular level could significantly enhance the evaluation of drug potency and safety profiles.
Moreover, the researchers envision further refinements to the technique to accurately quantify additional biomarkers such as DNA and RNA. Such advancements could elucidate fundamental biological questions—like discerning the specific biomolecular signatures associated with cell viability, whether a cell is thriving or in distress.
Future Directions and Impact on Cell Biology
One particularly intriguing application of the SAC-IR method is its potential role in cellular preservation techniques. A common practice in biotechnology involves freezing cells to maintain viability over extended periods. However, the optimal method for cell thawing and maintaining viability is still unclear. By harnessing the capabilities of infrared imaging, researchers hope to refine the freezing and thawing processes, optimizing conditions that preserve cellular integrity.
As the scientific community strives for more refined and rigorous methodologies to study the intricate world of cell biology, techniques like SAC-IR are proving invaluable. The ability to visualize and quantify biomolecules in living systems without invasive procedures is a game-changer, expediting research and potentially revolutionizing therapeutic approaches to diseases.
Innovations in biomolecular imaging, such as the SAC-IR technique developed by NIST, exemplify the strides being made to transform biotechnology. By overcoming water’s limiting factors through intelligent optical design, researchers can explore the living cell with unprecedented accuracy. As this method continues to evolve, it promises to forge new paths in our understanding of cellular dynamics, ultimately enhancing the efficacy of biotechnological advancements in medicine and beyond. The future is bright for researchers armed with these new tools, as they seek to unravel the complexities of life at the molecular level.