Cryopreservation stands at the forefront of advancements in medical science, playing a pivotal role in preserving critical biological materials like vaccines, blood donations, and fertility treatments. The need for effective freezing methods is underscored by the sensitive nature of biological materials that can degrade if not frozen quickly and properly. To address these challenges, researchers aim to enhance the processes involved in cryopreservation, thereby improving the usability and longevity of various medical treatments. While traditional methods have largely depended on trial and error, recent innovations promise to usher in a new methodological paradigm.
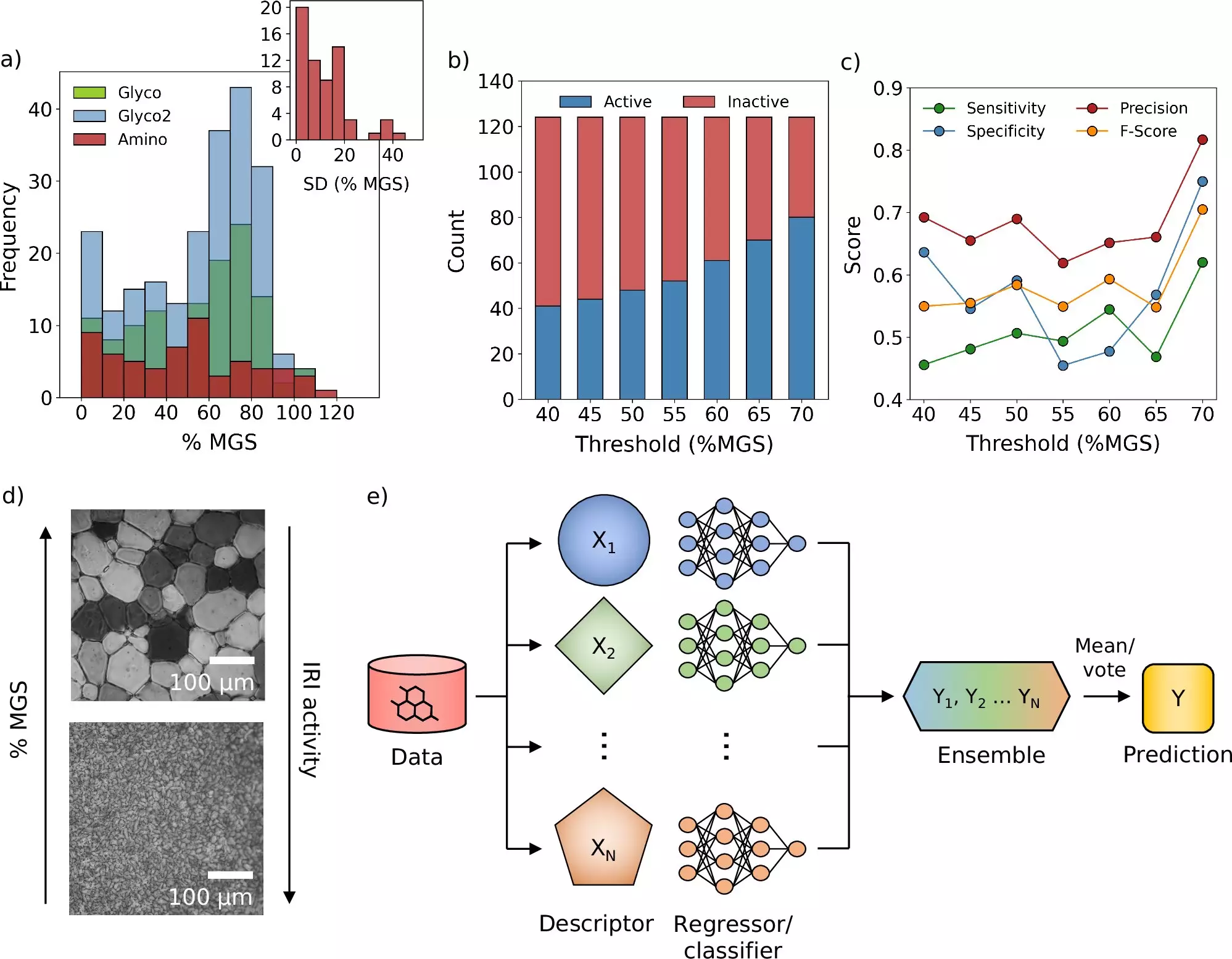
A recent collaboration between scientists at the University of Warwick and the University of Manchester has brought forth a groundbreaking computational model that significantly refines the freezing process for medicines and vaccines. This research, heralded for its innovative use of machine learning, allows for thousands of potential cryoprotective molecules to be evaluated through virtual simulations. This analytical framework not only reduces the time and resources expended in laboratory experiments but also provides an efficient avenue for identifying new compounds that could mitigate ice crystal formation during freezing—a significant hurdle in the cryopreservation process.
Prof. Gabriele Sosso emphasized that machine learning, while transformative, must be regarded as an adjunct to traditional scientific inquiry. It’s a tool among others that, when integrated with molecular simulations and empirical experiments, can yield remarkable results. This acknowledgment of the collaborative nature of science serves to remind us that while automation and artificial intelligence can augment human capabilities, they do not replace the essential creative and analytical human element in research.
One of the primary challenges in cryopreservation is the growth of ice crystals during the freezing and thawing processes. These crystals can cause significant damage to cells, undermining the efficacy of preserved therapies. Through their innovative computational framework, the research team has unearthed a novel molecule that displays the potential to significantly impede ice formation. This discovery is particularly consequential, as existing cryoprotectants have limitations; they primarily shield cells from damage but do not specifically address ice crystallization.
By employing a data-driven approach, the researchers were able to sift through extensive libraries of chemical compounds to pinpoint candidates ideal for cryopreservation applications. Dr. Matt Warren highlighted the excitement generated by using machine learning to optimize cryoprotectant selection, which has the potential to streamline laboratory workflows and refocus researchers’ efforts on intricate challenges that still require human introspection and creativity.
As promising as these findings may be, their tangible effects can be observed in practical experiments with blood samples. By incorporating the newly identified compounds, the team demonstrated that the quantity of conventional cryoprotectants necessary for blood storage could be considerably minimized. This reduction could facilitate faster processing times for post-freezing blood donations, expediting transfusion and potentially saving lives.
This groundbreaking work does not merely point toward the discovery of novel cryoprotectants but also shines a light on existing compounds that could be adapted for more efficient ice growth inhibition. Collaborations like those between Prof. Sosso and Prof. Matthew Gibson, who has researched ice-binding proteins in polar fish, are crucial in advancing the field. Their mutual expertise demonstrates how multidisciplinary partnerships can propel scientific discovery, revealing active molecules that might have otherwise remained overlooked.
What this research exemplifies is not merely incremental progress but rather a systemic shift in how scientific inquiries are conducted. By capitalizing on technological advancements in machine learning and computational modeling, researchers are positioned to redefine established protocols and uncover new frontiers in drug preservation.
Ultimately, these innovations have the potential to revolutionize not only cryopreservation but also a variety of biomedical applications, leading to more robust and versatile treatment modalities. As we stand on the cusp of these advancements, the collaborative spirit of science, which integrates technology with empirical discovery, will undoubtedly fuel future breakthroughs in the medical realm.