Hydrogen energy has emerged as a beacon of hope in addressing the pressing global energy crisis. Known for its clean and low-carbon emissions, hydrogen boasts a remarkably high calorific value, making it an attractive alternative to traditional fossil fuels. Among various methods of hydrogen production, electrochemical water splitting is garnering attention due to its potential for sustainability. However, the efficiency of this process is significantly hindered by the sluggish kinetics associated with the oxygen evolution reaction (OER). To overcome these challenges, innovative strategies for developing efficient catalysts are essential.
The ongoing quest for efficient OER catalysts has led to significant scientific scrutiny in recent years. One promising avenue of research has focused on single-atom catalysts (SACs), which offer unique advantages over conventional catalysts due to their ability to maximize atomic efficiency. Nonetheless, the challenge lies in the limited performance of these SACs, primarily attributable to the kinetics of the OER. The solution involves optimizing the arrangement and density of single atoms within the catalyst material to foster beneficial interactions that can enhance their catalytic properties.
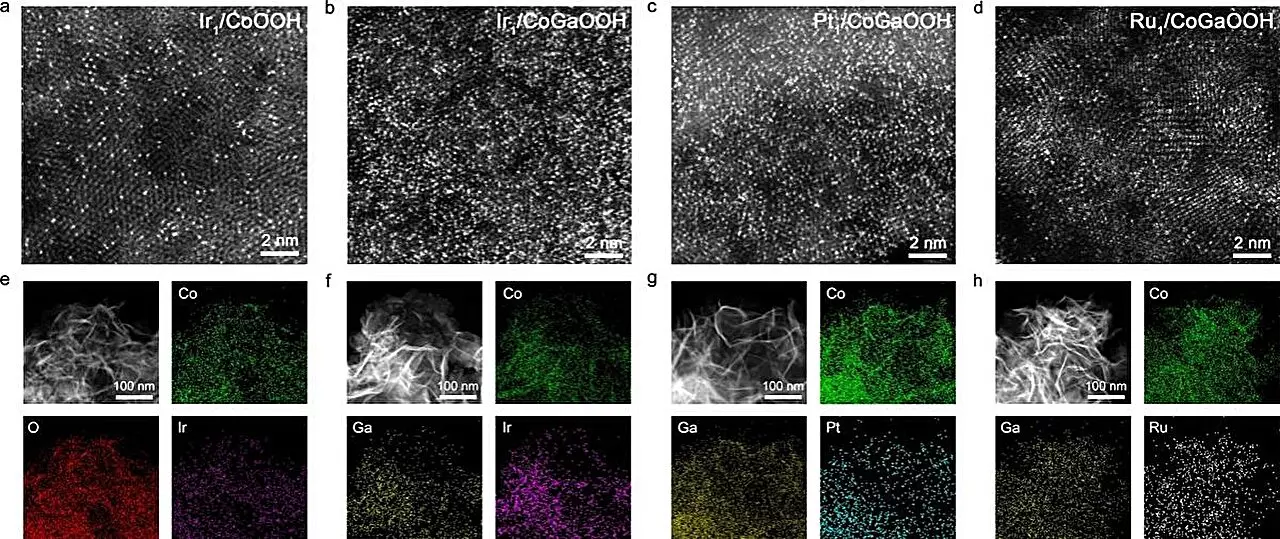
Recent research led by Prof. Bao Jun and his team at the University of Science and Technology of China (USTC) has made strides in addressing these issues. Their work focuses on a unique cobalt-based, oxide-supported high-density single-atom Ir catalyst. The innovation lies in creating a high density of single atoms, which facilitates neighboring synergetic interactions. As the proximity of these atoms increases, their ability to work together improves, significantly optimizing the kinetics of the OER. This novel finding was documented in a study published in the prestigious journal *Angewandte Chemie*.
The research team meticulously engineered these SACs to understand the implications of atom density. By incorporating gallium (Ga) atoms into the cobalt oxide lattice, they were successful in modulating the electronic characteristics of the single-atom sites. This precise fabrication was critical as it not only influenced the electronic structure but also enhanced the interaction strength between oxygen-defect sites and the precursor atoms.
The result of these meticulous advancements was a noteworthy catalyst designated as Nei-Ir1/CoGaOOH. The performance metrics of this catalyst are particularly striking; it demonstrated a low overpotential of just 170 mV at a current density of 10 mA cm-2. Additionally, it showcased remarkable stability, maintaining its operational efficacy over an extensive period, including maintaining stability at a current density of 1 A cm-2 in an alkaline environment for more than 50 hours.
In-situ studies, particularly Raman spectrometry, confirmed the structural integrity of the catalyst during operation, a promising indication of its practical applicability in real-world scenarios. These results reveal that optimizing the density of Ir single atoms not only improves performance metrics but also paves the way for long-term stability in electrochemical applications.
Mechanistic Insights Revealed
Diving deeper into the mechanics of the catalyst’s performance, the researchers revealed that the enhanced activity does not solely derive from modifications in electronic characteristics. Instead, it stems from the synergetic interactions among high-density Ir single atoms. These interactions effectively stabilize reaction intermediates like *OOH* through additional hydrogen-bonding interactions, which diminish the reaction energy barrier. This critical insight underscores the importance of atom proximity in catalysis and opens new avenues for optimizing SACs for improved efficiency.
The findings from Prof. Bao Jun and his research team illustrate a significant leap in our understanding of SACs within the realm of electrochemical reactions. By elucidating the mechanics behind high-density interactions, researchers now have a structured framework to develop next-generation catalysts designed to achieve high performance with stable operation over prolonged periods.
This groundbreaking research not only provides a clearer perspective on the functioning of SACs but also paves the way for future innovations in catalyst design aimed at tackling the challenges associated with the energy crisis. As researchers continue to explore and refine SAC technologies, the dream of efficient, clean hydrogen energy may soon transition from theoretical promise to practical reality.