The idea of self-assembly conjures visions of magically piecing together something intricate and functional from assorted components, much like a puzzle that completes itself. In the realm of chemistry, self-assembly is not only a concept but a fundamental process that pervades biological structures, enabling everything from protein formation to virus construction. The field known as supramolecular chemistry seeks to explore and exploit this phenomenon by creating large molecules from simpler, smaller units, which has profound implications for material science and biology alike.
Supramolecular chemistry stands at the forefront of innovative material design, employing a range of techniques to manipulate molecules at the nanoscale. The ability to control the interactions between various polymers allows scientists to design materials that respond to external stimuli. This is crucial in developing “smart materials” that can adapt their properties based on changes in their environment, such as chemical signals. Despite exciting advancements, the intricacies of these interactions still present significant challenges and gaps in comprehension.
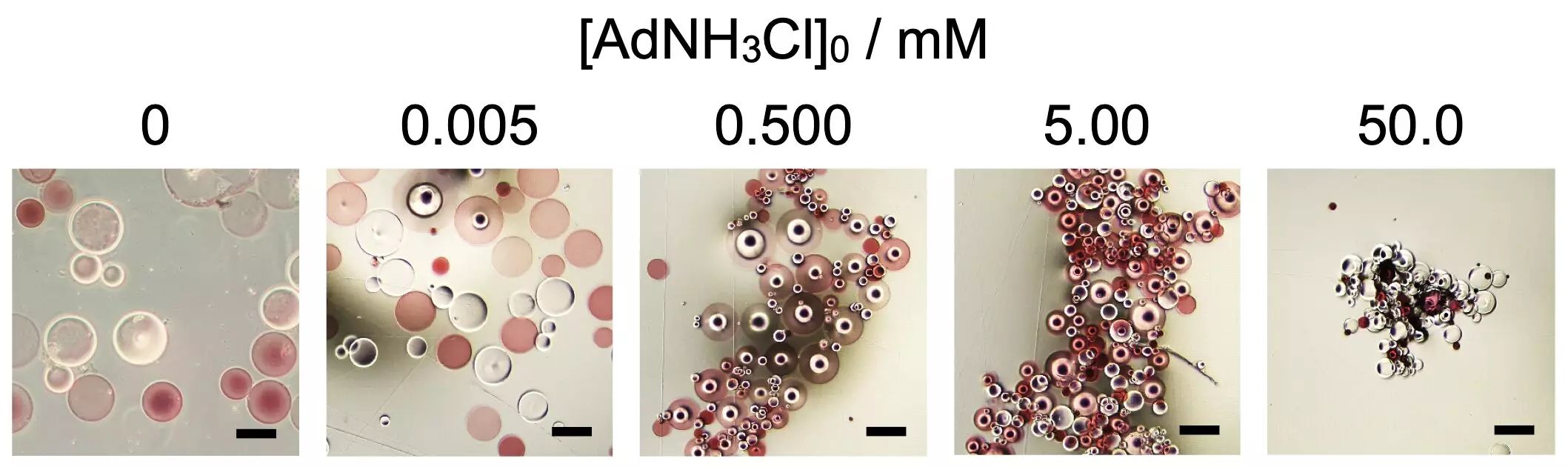
Recent findings from Osaka University shed light on the dynamic nature of self-assembly through the use of specific additives. The researchers discovered that by manipulating the concentration of the additive 1-adamantanamine hydrochloride (AdNH3Cl), they could influence the formation and shape of spherical microparticles made from the superabsorbent polymer poly(sodium acrylate). Noteworthy is how incorporating functional groups like beta-cyclodextrin and adamantane residues influenced the self-assembly process, revealing an intricate dance between chemistry and structure.
This experimental insight parallels the behavior of biological proteins, which are composed of amino acids interacting through various forces such as hydrogen bonds and electrostatic forces. These interactions dictate the folded structures of proteins essential for biological functionality. As noted by Akihito Hashidzume, the lead of the study, the principles underlying the self-assembly of these man-made polymers can indeed echo the complexities observed in living organisms.
The researchers also emphasized the significance of being able to manipulate the resulting shapes of the microparticle assemblies. By adjusting the concentration of AdNH3Cl, they could create either spherical or elongated structures, which could alter their properties and applications. This aspect opens new avenues for controlling macroscopic assemblies through intricate microscopic interactions, setting a stage for innovative applications in both materials science and biology.
As the senior author Akira Harada highlighted, this research not only demystifies the mechanisms behind self-assembly but also holds potential implications for understanding the various shapes and forms seen in organic life. Such knowledge could inform the development of new active materials that dynamically respond to their environment—an exciting frontier that combines the creativity of design with the rigor of scientific inquiry.
The intersection of supramolecular chemistry with biological principles provides an enriching framework for understanding self-assembly’s role in nature and technology. As researchers continue to untangle these complex interactions, the applications envisioned could redefine how we approach materials, paving the way for smarter, more adaptive structures that mirror the remarkable efficiency found in biological systems. The journey has just begun, and the potential is immense.